Types of content
-
Signposting information or directly communicating?
It is important to differentiate between ‘disseminating information directly’ and ‘signposting to information’. The difference is critical to engaging with stakeholders in social media compliantly. Signposting must be used when the information being signposted to is intended only for a specified audience.
‘Signposting’ points to information while:
• making clear who the information is for
• indicating the nature of the information
• requiring the user to take active steps to access further information.Depending on the content that is being signposted to, the individual may need to confirm their status prior to accessing further information (e.g. requiring an individual to confirm they are a health professional before registering for a promotional meeting) or validation of the individual by the pharmaceutical company (e.g. upon joining a closed user group).
The information in the ‘signpost’:
• must be sufficient to enable the viewer to determine whether the information is relevant to them and to choose to find out more
• must be appropriate for the public
• must not constitute the promotion of a prescription only medicine to the public
• must not constitute the promotion of an unlicensed medicine.For example, signposting can be used to invite health professionals to register for a promotional meeting:
- The signpost must indicate that the meeting is for UK health professionals only.
- The signpost must indicate that the meeting will include product promotion.
- The information provided in the signpost must not directly or indirectly reference a specific medicine.
- The signpost can link to a meeting registration webpage which clearly states the intended audience. The reader is required to self-attest as a health professional before accessing this page. If the individual does not confirm they are a health professional, they must be redirected to suitable information for the public (e.g. homepage of corporate website or a suitable NHS page).
- More formal validation of health professionals during registration/prior to meeting (e.g. requiring a healthcare organisation email address, location and job title) might be required to ensure only appropriate attendees attend the meeting itself.
- It is important that the intended audience is very clear at all times, including on the original social media post.
- Companies should consider limiting the audience of the post using targeting criteria where possible.
Example of what might be acceptable:

Relevant cases to read (non-exhaustive list):
For guidance on signposting information for an investor audience and the media, see ‘News and announcements for an investor audience and the media’ below.
Last revised: 2 February 2026
- The signpost must indicate that the meeting is for UK health professionals only.
-
Promotion to health professionals and other relevant decision makers
Prescription only medicines must not be advertised to the public so promotional activities on social media, including paid-for advertising, must be carefully targeted to relevant health professionals only.
In addition, pharmaceutical companies should check the current terms and conditions for the relevant social media platform to ensure its activities comply with the platform’s rules as well as all applicable codes, laws and regulations.
Due to UK legal requirements and the open and transitory nature of these social media platforms, pharmaceutical companies should consider very carefully before engaging in and facilitating discussions about medicinal products on these channels.
It is important to consider the reach of the channel/platform and the ability for the company to control who can and cannot receive the information being provided. Pharmaceutical companies should consider what controls could be put in place to restrict the sharing of such promotion on social media platforms to avoid content being re-shared with other audiences, such as members of the public.
It is also important that pharmaceutical company involvement is clear from the outset and all other obligatory requirements in relation to promotion to health professionals are met, such as the provision of prescribing information, the adverse event reporting statement and obtaining prior permission from the health professionals to receive promotional material in this way as required by Clause 15.5.
Pharmaceutical companies should also bear in mind Clause 5.7 of the ABPI Code which states that material should only be provided or made available to those groups of people whose need for or interest in it can reasonably be assumed and the material should be tailored to the audience to whom it is directed.
Annex 2 ‘Principles for the use of digital channels’ of the EFPIA Code states that the use of social media directed to the public to alert health professionals about the publication of a study on a medicinal product is also likely to be considered promotion of that medicinal product and is therefore prohibited.
Could a non-promotional post link to a promotional website with an HCP-only gateway?
The PMCPA considers each case on its own merits. Companies should bear in mind Clause 5.7 which states that material should only be provided or made available to those groups of people whose need for or interest in it can reasonably be assumed and that material should be tailored to the audience to whom it is directed. Promotional materials and activities must not be disguised. In some instances, you may be able to use ‘signposting’; however, you will have to use all the safeguards that apply to signposting as referred to in the 'Signposting information or directly communicating?' section (above), including the use of targeting criteria.
It is also worth bearing in mind the supplementary information to Clause 16.1 which states that unless access to promotional material about prescription only medicines is limited to health professionals and other relevant decision makers, a pharmaceutical company website or a company sponsored website must provide information for the public as well as promotion to health professionals with the sections for each target audience clearly separated and the intended audience identified. This is to avoid the public needing to access material for health professionals unless they choose to. The MHRA Blue Guide states that the public should not be encouraged to access material which is not intended for them.
Relevant cases to read (non-exhaustive list):
Last revised: 2 February 2026
-
News and announcements suitable for the general public
Pharmaceutical companies can post or share/disseminate non-product related company news on social media. The news that is shared/disseminated must be appropriate for the general public and must not directly or indirectly refer to a specific medicine. News can include, for example, new executive appointments, new manufacturing facilities, employee recognition, and company awards.
Last revised: 2 February 2026
-
News and announcements for an investor audience and the media
The supplementary information to Clause 26.2 of the ABPI Code ‘Financial Information’ states that information made available in order to inform shareholders, the Stock Exchange and the like by way of annual reports and announcements, etc. may relate to both existing medicines and those not yet marketed. Such information must be non-promotional, accurate, presented in a factual and balanced way and not misleading, taking into account the information needs of the target audience. Business press releases should identify the business importance of the information and should only be aimed at the intended financial and investment audience.
Typically, information for investors and journalists is sent directly to these audiences and provided in a publicly accessible section of a pharmaceutical company’s corporate website that is clearly labelled with the intended audience.
Increasingly pharmaceutical companies wish to use social media to inform investors or prospective investors and/or appropriate journalists of newsworthy information as well as significant changes affecting company investor outlook. Using social media channels to communicate this information is complex as by their nature social media channels are open to a broad audience, beyond the intended audience for the post itself.
It is important to differentiate between ‘disseminating information directly’ and ‘signposting to information’. The difference is critical to engaging with an investor audience/journalists on social media compliantly. Signposting must be used when the information being signposted to is intended only for an investor audience and the media.
‘Signposting’ points to information while:
• making clear who the information is for
• indicating the nature of the information
• requiring the user to take active steps to access further information.Pharmaceutical companies should consider the following guidance and safeguards when considering posts for an investor audience/journalists on social media:
- The information within the post and linked content being signposted to should be newsworthy bearing in mind the intended audience.
- The material should be tailored to the intended audience.
- No self-attestation of investor/media status required, however, both the post and the content it links to should have intended audience prominently signposted at the outset.
- Any information provided must be factual, balanced and must not encourage members of the public to ask their doctors or other prescribers to prescribe a specific prescription only medicine. It must not constitute the advertising of prescription only medicines to the public. Neither must it constitute the promotion of an unlicensed medicine or indication.
- Any mention of a medicine in a social media post is likely to be considered promotion of that medicine to the public and is prohibited. Therefore, the body of the social media post itself should not name (brand or non-proprietary) the medicine. Note, this is consistent with Annex 2 of the EFPIA Code 'Principles for the use of Digital Channels’.
- The linked content (e.g. press release, annual report, video of financial results etc.) can name medicines, but this must be in accordance with the requirements of Clause 26.2 and its supplementary information and must require the reader to take active steps to view e.g. click on a link. There must be no autoplay of content that names a medicine.
- Only social media accounts likely to be followed by an investor audience/the media should publish posts intended for this audience i.e. the company corporate account or the accounts of its senior executives (CEO, CFO, COO or similar). Care must be taken as excessive social media activity about the same news might constitute promotion.
In certain scenarios, a distinction might be made between a medicine being indirectly referenced versus promoted. E.g. a specific medicine might be identifiable from a post without its name being mentioned if there is only one medicine for the condition referred to in the post. Context and use of language will be considered carefully in the event of a complaint.
Examples of what might be acceptable
Communication of financial results/annual reports
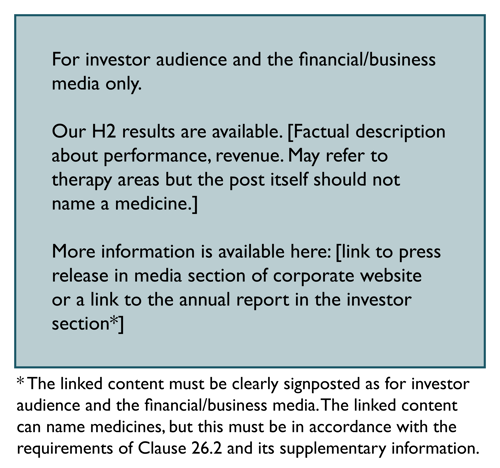
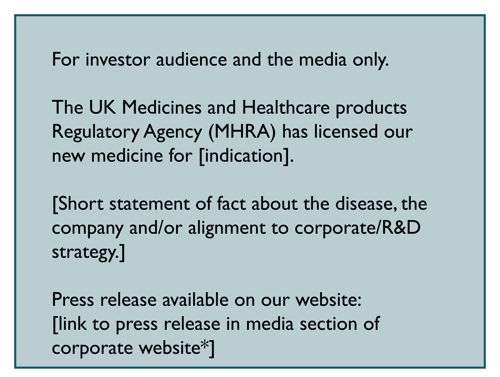
New product authorisations

New major published data

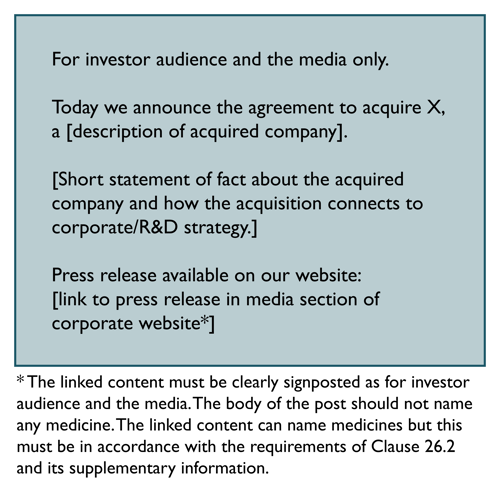
New company acquisition
Last revised: 2 February 2026
- The information within the post and linked content being signposted to should be newsworthy bearing in mind the intended audience.
-
Does the prohibition on medicine name in a post apply to investigational compounds with no INN?
Clause 3.1 states that a medicine must not be promoted prior to the grant of the marketing authorisation which permits its sale or supply.
UK law only refers to medicines with or without a marketing authorisation with no further distinction.
There is no defined time point at which a compound from discovery research is considered an unlicensed medicine for the purposes of the Code, and this will need to be considered on a case-by-case basis, taking all factors into consideration.
A compound may be considered an unlicensed medicine as early as when clinical trials have commenced and thus Clause 3.1 could become engaged from this time.
The Panel’s rulings give an indication of the factors considered in each case. Each case is assessed on its individual merits.
Based on case precedent, if clinical trials have not commenced or if the compound is still only known by its developmental code number and has not yet been given a non-proprietary name (INN), then it might not be considered a medicine for the purposes of Clause 3.1. In such a case, the compound could be directly referred to within a factual, balanced, accurate and non-misleading social media post.
Relevant cases to read (non-exhaustive list):
Last revised: 2 February 2026
-
Disease awareness for the public
Disease awareness can be conducted by a pharmaceutical company via social media provided that the purpose is to increase awareness of a disease/health condition and encourage members of the public to visit their health professional to seek treatment/management of their symptoms while in no way promoting the use of a specific medicine. The use of brand or non-proprietary names and/or restricting the range of treatments described in the campaign might be likely to lead to the use of a specific medicine. Particular care must be taken where the company’s product, even though not named, is the only medicine relevant to the disease or symptoms in question. Any websites or other materials linked to a social media post to promote disease awareness must also be non-promotional.
Attention is drawn to the MHRA Blue Guide Appendix 7, Disease Awareness Campaign Guidelines.
Companies must bear in mind the certification requirements of Clause 8.3.
Companies may allow staff to share company disease awareness posts intended for the public if that post has been certified as required by the ABPI Code. Staff should be instructed not to add their own commentary when sharing the material. Furthermore, certain interaction by staff with the original post, e.g. comments below the original post appearing on the company account, may change the meaning of the post and therefore should be avoided.
Example of what might be acceptable: Posts on Facebook or X about a disease awareness campaign directing people to a dedicated disease-orientated Facebook page or website for further information. Both the post and the linked website must meet the requirements of the Code for disease awareness.
Does a social media post certified under Clause 8.3 need to be deleted if it is not re-certified within two years?
Clause 8.3 requires that educational material for the public or patients issued by companies which relates to diseases or medicines but is not intended as promotion for those medicines, is certified in advance in a manner similar to that provided for by Clause 8.1.
Clause 8.5 states that material which is still in use must be recertified at intervals of no more than two years to ensure that it continues to conform with the relevant regulations relating to advertising and the Code.
Companies should assess the purpose of keeping posts that are older than two years on their social media accounts. Companies should treat their social media accounts similarly to their corporate websites in terms of oversight.
Not all social media posts made by a company will require certification under the Code; however, posts that have required certification – for example, disease awareness posts – should be re-certified within two years or removed from the company’s social media account. Through the process of re-certification, companies will be checking whether the information is still accurate, balanced and up to date.
It is important to remember that corporate advertising, press releases, financial information to inform shareholders and the stock exchange, etc. do not need to be certified under the Code (See Clause 8.3 Examination of Other Material). If companies choose to certify material that does not require certification under the Code, there is no subsequent requirement to re-certify it. Nonetheless, as with all materials, companies should maintain adequate oversight.
What about company staff who have interacted with posts certified under Clause 8.3 where the original post is subsequently deleted by the company. Does the company need to ask its staff to also delete the post?
Pharmaceutical companies should have policies on how staff may interact with company posts. Companies may allow staff to share a disease awareness post intended for the public if that post has been certified as required by the ABPI Code. Staff should be instructed not to add their own commentary when sharing the material.
Companies should also be aware that certain interaction by staff with the original post, e.g. comments below the original post appearing on the company account, may change the meaning of the post and therefore should be avoided.
Companies could consider turning off comments on the content and limiting its engagement to a short, predefined period. If comments remain enabled, companies should be aware of the risk that comments may result in inappropriate content appearing alongside a company post. When comments are enabled, they should always be actively monitored.
If the company subsequently deletes the original post from its corporate account, some forms of interaction with it by others may be automatically deleted from the platform. However, there will be some forms of interaction that effectively created a new post and therefore the information may still be available.
If a company removes the original post because the two-year certification period has elapsed, it is not expected to ask its personnel to delete their past interaction with it if such interaction had created a new post. However, if the post is being withdrawn by the company due to an error or breach of the Code, the company should take all possible steps to have personnel (including its third parties) delete the post too.
Relevant cases to read (non-exhaustive list):
Last revised: 2 February 2026
-
Patient support
The ABPI Code distinguishes between the public and patients prescribed a particular medicine in certain instances but there is no such distinction in UK law.
Pharmaceutical companies can use social media to host information for patients who have been prescribed a specific prescription only medicine, such as videos hosted in the non-public sections of social media platforms like YouTube (i.e. cannot be found by a general search). The target audience should be clearly identified and the content appropriate for that audience.
Example of what might be acceptable: Video about how to take a medicine correctly, developed by a company and hosted as an unlisted video (i.e. cannot be found by a general search) on YouTube with a URL which can be shared with patients who have been prescribed the medicine and clearly identifies the target audience as those who have been prescribed the medicine.
Last revised: 2 February 2026
-
Job adverts
Social media platforms can be used by employers to find candidates and by job seekers to apply for jobs.
Titles in job advertisements should avoid mentioning prescription only medicines as this may constitute promotion of a prescription only medicine to the public. It is particularly likely to constitute promotion if the medicine is named alongside the product indication, therapy area, or key product benefits. A poorly worded post about the role that was proactively distributed to many people, is likely to be considered to be promotion of a prescription only medicine to the public.
It might be permissible to include in an appropriate and proportionate way, the medicine name within the more detailed content of the job advertisement if it was relevant to job seekers and if it would involve additional clicks and/or scrolling by the reader to view the information.
Last revised: 2 February 2026
-
Clinical trial recruitment
A memorandum of understanding (MOU) between the ABPI, the PMCPA and the Health Research Authority (HRA) came into effect on 1 February 2026.
Part of the HRA’s role, in collaboration with the Devolved Authorities, is to administer the NHS Research Ethics Committees (RECs) that exist across the UK.
RECs review research proposals and give an opinion about whether the research is ethical. They also review participant-facing material, including clinical trial recruitment material. It is a REC’s responsibility to determine whether public-facing information it is considering is ethical, including whether that information is non-promotional, non-coercive and not misleading as to the potential benefit.
REC-approval of participant-facing clinical trial recruitment material, including advertisements on social media, is a legal requirement.
The MOU states that participant-facing clinical trial recruitment material that has been REC-approved will be regulated and adjudicated upon by the HRA; not the PMCPA. It is also important that questions and/or concerns about clinical trial recruitment material, including on social media, are directed to the HRA given its specific regulatory remit in this area.
Guidance and responses to queries in relation to clinical trial recruitment material will be provided by the HRA. Queries should be sent to approvals@hra.nhs.uk.
As REC-approval of participant facing clinical trial recruitment advertisements on social media is a UK legal requirement, and regulated by the HRA, companies are not required to certify such posts under Clause 8.3.
Last revised: 2 February 2026
