Responsibility
-
Who is responsible under the ABPI Code for social media activity?
Company social media activity
A company owning the social media account is responsible for the content and any mention of a prescription only medicine is likely to be considered promotion of that medicine to the public and is prohibited. This provision is reflected in Annex 2 of the EFPIA Code ‘Principles for the use of digital channels’.
A pharmaceutical company is responsible for all material disseminated/activities carried out by it, on any social media channel, that comes within the scope of the ABPI Code. It is important that companies have regular oversight of all their social media accounts, including when staff managing the accounts leave the company.
Pharmaceutical companies also have responsibilities when engaging with social media accounts owned by other companies or organisations. If a UK company engages with a post (for example by ‘liking’ or re-sharing), it would be subject to the ABPI Code if the content was within the scope of the Code and the engagement disseminated the information. The latter would be dependent on the functionality of the social media platform but, in most cases, by engaging with a post the content is disseminated to the account’s own connections/followers. Any commentary on another account's post that was within the scope of the Code would also be subject to the Code, regardless of whether the activity disseminated the information further.
Relevant cases to read (non-exhaustive list):
Social media activity by third parties
Pharmaceutical companies are responsible under the Code for the acts and omissions of their third parties which come within the scope of the Code, even if they act contrary to the instructions which they have been given.
Clause 1.24 defines a third party as a legal person/entity or individual that represents a company or interacts with other parties on behalf of a company or relating to a company’s medicine, such as distributors, wholesalers, consultants, contract research organisations, professional congress organisers, contracted sales forces, market research companies, advertising agencies, media buyers, providers of services related to events, public relations services, non-clinical services, non-interventional studies management services, etc.
Pharmaceutical companies should ensure that third parties, including consultants such as contracted speakers/influencers, are aware of the requirements of the ABPI Code, particularly in relation to social media and the prohibition on advertising prescription only medicines to the public.
Written agreements with third parties should deal comprehensively with ownership and control, including use of and potential withdrawal of materials both during and after the contractual period.
Pharmaceutical companies are strongly advised to preview social media content from their contracted parties in relation to their contracted activities and, of course, are responsible for certification of these posts when required by the ABPI Code.
A pharmaceutical company may also be responsible for material/activities sponsored by it, depending on the sponsorship arrangements.
Relevant cases to read (non-exhaustive list):
Personal use of social media
The personal use of social media by pharmaceutical company staff (employees and contractors) and the staff of third parties has the potential to overlap with their professional responsibilities. Company staff should act with due caution when using all social media platforms, including LinkedIn, to discuss or highlight issues which relate to their professional role or the commercial/research interests of their company. Staff of third parties should be cautious when engaging with content related to their client or their client’s commercial/research interests.
On most social media platforms, an individual’s personal activity on social media will, in the first instance, be visible to their own connections/followers. It will potentially also be visible to others outside their network, depending on the individual’s security settings. Individuals should assume that their activity would, therefore, potentially be visible to both health professionals or other relevant decision makers and to members of the public.
If personal use of social media was found to be in scope of the ABPI Code, the pharmaceutical company would be held responsible.
Pharmaceutical companies may be held responsible for engagement with, or dissemination of, information by individuals who do so via their personal social media channels including:
(a) if the individual can reasonably be perceived as representing the company, and/or
(b) if the individual is instructed, approved, or facilitated by the company to do so.
Pharmaceutical companies should:
• assume that the ABPI Code would apply to all work-related, personal social media activity by their staff unless, for very clear reasons, it could be shown otherwise
• ensure that they have appropriate policies in place and staff receive regular training appropriate to their role
• ensure third-parties are aware that their staff should be cautious about engaging with content relating to their client or their client’s commercial/research interests.There are no exceptions to the applicability of the ABPI Code depending on which part of the business, e.g. finance, IT, manufacturing, medical or commercial, the individual who has issued or engaged with the post works.
Relevant cases to read (non-exhaustive list):
- Case/0587/05/25
- Case AUTH/3926/6/24
- Case AUTH/3879/2/24
- Case AUTH/3867/12/23
- Case AUTH/3835/10/23
- Case AUTH/3758/4/23
Last revised: 2 February 2026
-
When does social media activity outside the UK fall within the scope of the ABPI Code?
For social media activity to fall within the scope of the ABPI Code, there must be a UK nexus. The account, content and intended geographical audience may be relevant when determining whether there is a UK nexus.
Clause 1.2 of the ABPI Code states:
“Information or promotional material about medicines which is placed on the internet outside the UK will be regarded as coming within the scope of the Code, if it was placed there by:
• a UK company/with a UK company’s authority, or
• an affiliate of a UK company, or with the authority of such a company, and it makes specific reference to the availability or use of the medicine in the UK.”Clause 16.1 of the ABPI Code states:
“Promotional material about prescription only medicines directed to a UK audience which is provided on the internet must comply with all relevant requirements of the Code.”
It is an established principle under the ABPI Code that UK pharmaceutical companies are responsible for the activities of overseas affiliates where those activities come within the scope of the ABPI Code.
Companies can use the traffic light system below to help them decide whether the activity is within the scope of the ABPI Code. If the activity is not within scope of the ABPI Code, it may be in scope of another country's code, laws or regulations. An activity may also be in scope of multiple country codes, laws and regulations.
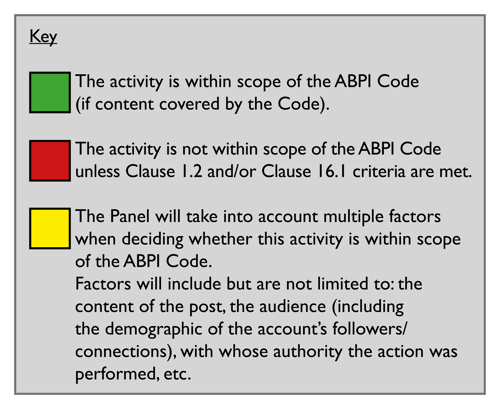
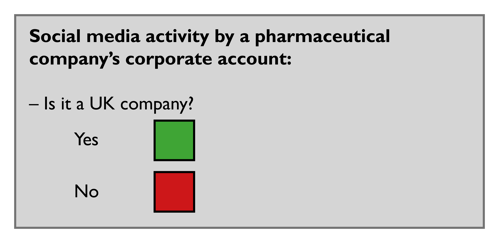

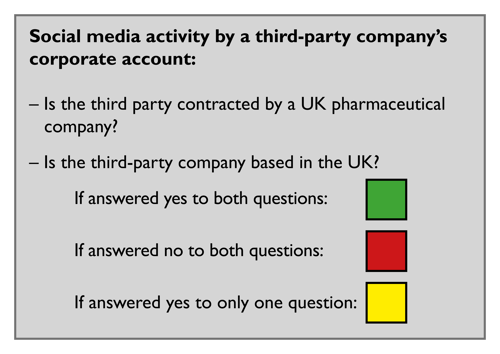

Relevant cases to read (non-exhaustive list):
- Case/0496/03/25
- Case/0224/07/24
- Case AUTH/3894/4/24
- Case AUTH/3810/8/23
- Case AUTH/3805/7/23
- Case AUTH/3775/6/23
- Case AUTH/3747/2/23
- Case AUTH/3743/2/23
- Case AUTH/3741/2/23
- Case AUTH/3707/11/22
- Case AUTH/3623/3/22
- Case AUTH/3579/11/21
- Case AUTH/3444/12/20
- Case AUTH/3430/11/20
Last revised: 2 February 2026
