Case Summary
A complainant who described him/herself as a concerned UK health professional, complained about an advertisement for fluoxetine issued by Endo Ventures. Fluoxetine’s indications included the treatment of major depressive episodes in adults. The advertisement included a claim for fluoxetine ‘for treatment of depressive illness’.
The complainant alleged that ‘depressive illness’ as referred to in the advertisement was broader than the licensed indications.
The detailed response from Endo Ventures is given below.
The Panel noted that fluoxetine was indicated for adults for major depressive episodes, obsessive compulsive disorder or bulimia nervosa and for children and adolescents aged 8 years and above for moderate to severe major depressive episodes. The Panel noted that these indications were given in the prescribing information in the advertisement as were details about the age range for use of the medicine. The first mention of an indication was in the advertisement where the claim ‘for treatment of depressive illness’ appeared below the product name. The Panel considered that the claim was not an accurate reflection of any of the fluoxetine indications and was therefore inconsistent with the SPC. The Panel ruled a breach of the Code as acknowledged by Endo Ventures.
The Panel considered that high standards had not been maintained and ruled a further breach.
CASE AUTH/3149/1/19 ANONYMOUS COMPLAINANT v ENDO VENTURES
Promotion of Fluoxetine
A complainant who described him/herself as a concerned UK health professional, complained about an advertisement for fluoxetine issued by Endo Ventures. Fluoxetine’s indications included the treatment of major depressive episodes in adults. The advertisement included a claim for fluoxetine ‘for treatment of depressive illness’.
The complainant alleged that ‘depressive illness’ as referred to in the advertisement was broader than the licensed indications.
The detailed response from Endo Ventures is given below.
The Panel noted that fluoxetine was indicated for adults for major depressive episodes, obsessive compulsive disorder or bulimia nervosa and for children and adolescents aged 8 years and above for moderate to severe major depressive episodes. The Panel noted that these indications were given in the prescribing information in the advertisement as were details about the age range for use of the medicine. The first mention of an indication was in the advertisement where the claim ‘for treatment of depressive illness’ appeared below the product name. The Panel considered that the claim was not an accurate reflection of any of the fluoxetine indications and was therefore inconsistent with the SPC. The Panel ruled a breach of the Code as acknowledged by Endo Ventures.
The Panel considered that high standards had not been maintained and ruled a further breach.
A complainant who described him/herself as a concerned UK health professional, complained about an advertisement for fluoxetine (ref FLUO 0010/2018) which was placed in Pulse by Endo Ventures Ltd. Fluoxetine’s indications included the treatment of major depressive episodes in adults. The advertisement included a claim for fluoxetine ‘for treatment of depressive illness’.
COMPLAINT
The complainant alleged that ‘depressive illness’ as referred to in the advertisement was broader than the licensed indications which were:
‘Adults: Major depressive episodes Obsessive-compulsive disorder Bulimia nervosa: Fluoxetine is indicated as a complement to psychotherapy for the reduction of binge-eating and purging activity.
Children and Adolescents aged 8 years and above: Moderate to severe major depressive episode, if depression is unresponsive to psychological therapy after 4-6 sessions. Antidepressant medication should be offered to a child or young person with moderate to severe depression only in combination with a concurrent psychological therapy.’
The complainant alleged that the advertisement appeared to promote fluoxetine off-licence both in terms of the age of the individual as well as the severity of the depression.
When writing to Endo Ventures, the Authority asked it to consider the requirements of Clauses 3.2 and 9.1 of the 2016 Code.
RESPONSE
Endo Ventures explained that to ensure compliance with the Code and MHRA Blue Guide, it had engaged the services of a third party to advise it with regard to promotional activities and, in particular, to provide medical approval for promotional materials including advertisements. A copy of the approval certificate, signed by the third party, was provided.
On receipt of the complaint, the third party signatory reviewed the advertisement and acknowledged that the claim ‘for treatment of depressive illness’, in isolation, could be considered as too broad a representation of the indication. However, the full licensed indications, as listed in the summary of product characteristics (SPC), were included as an integral part of the advertisement in the prescribing information on the same page.
Regarding the advertisement as a whole, it was clear that there was no intent to promote offlabel. However, in consideration of this feedback, Endo Ventures had immediately withdrawn all future advertisements using the claim at issue and undertook that future advertisements would contain a modified statement of the indication, for example, ‘for the treatment of major depressive episodes in adults’.
In response to the complainant’s comment that the advertisement appeared to promote the product offlabel both in terms of the age of the individuals and the severity of depression, Endo Ventures repeated that it had no intention to promote off-label, and the text extracted by the complainant from the prescribing information was a true representation of the indications in the SPC. A side-by-side comparison of the text in the SPC and prescribing information was as follows:
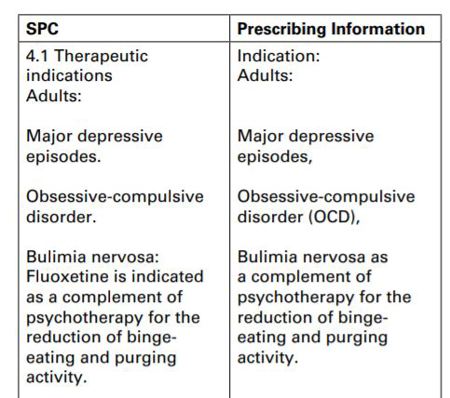

In conclusion, Endo Ventures stated that it had followed due process in clearing the advertisement through its internal review process via an established external regulatory consultancy, which approved the advertisement at issue. Endo Ventures’ intention had always been to operate in compliance with the Code. Following receipt of the complaint and having reviewed the objection with regards to the claim ‘for treatment of depressive illness’, Endo Ventures agreed to amend this to ‘for the treatment of major depressive episodes in adults’ in all future versions of this advertisement.
PANEL RULING
The Panel noted that fluoxetine was indicated for adults for major depressive episodes, obsessive compulsive disorder or bulimia nervosa and for children and adolescents aged 8 years and above for moderate to severe major depressive episodes. The Panel noted that these indications were given in the prescribing information in the advertisement as were details about the age range for use of the medicine. The first mention of an indication was in the advertisement where the claim ‘for treatment of depressive illness’ appeared below the product name. The Panel considered that the claim was not an accurate reflection of any of the fluoxetine indications and was therefore inconsistent with the SPC. The Panel ruled a breach of Clause 3.2 as acknowledged by Endo Ventures.
The Panel considered that high standards had not been maintained and ruled a breach of Clause 9.1.
Complaint received 9 January 2019
Case completed 30 April 2019


