CASE AUTH/2670/11/13 ANONYMOUS CONTACTABLE MEMBER OF THE PUBLIC v OTSUKA
NO BREACH OF THE CODE
Clinical trial disclosure (Samsca)
An anonymous, contactable member of the public complained about the information published as ‘Clinical Trial Transparency: an assessment of the disclosure results of company-sponsored trials associated with new medicines approved recently in Europe’. The study was published in Current Medical Research & Opinion (CMRO) on 11 November 2013.
The study authors were Dr B Rawal, Research, Medical and Innovation Director at the ABPI and B R Deane, a freelance consultant in pharmaceutical marketing and communications. Publication support for the study was funded by the ABPI.
The study surveyed various publicly available information sources for clinical trial registration and disclosure of results searched from 27 December 2012 to 31 January 2013. It covered 53 new medicines (except vaccines and fixed dose combinations) approved for marketing by 34 companies by the European Medicines Agency (EMA) in 2009, 2010 and 2011. It included all completed company-sponsored clinical trials conducted in patients and recorded on a clinical trial registry and/or included in a European Public Assessment Report (EPAR). The CMRO publication did not include the specific data for each product. This was available via a website link and was referred to by the complainant. The study did not aim to assess the content of disclosure against any specific requirements.
The complainant stated that the study detailed a number of companies which had not disclosed their clinical trial results in line with the ABPI for licensed products. The complainant provided a link to relevant information which included the published study plus detailed information for each product that was assessed.
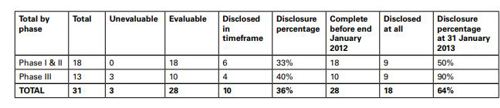
The summary output for each medicine set out the sources for all trials found, irrespective of sponsor and an analysis of publication disclosure in the form of a table which gave details for the studies for Samsca (tolvaptan).
The detailed response from Otsuka is given below.
General detailed comments from the Panel are given below.
The Panel noted the CMRO publication in that 18 evaluable studies had not been disclosed within the timeframe. The disclosure percentage was 36%. Ten studies completed before the end of 2012 had not been disclosed. The disclosure percentage at 31 January 2013 of trials completed before the end of January 2012 was 64%. A footnote stated that the undisclosed Phase I/II trials comprised of trials completed before reporting requirements. Trials with no US IND therefore not subject to FDAAA 801 requirements. The undisclosed Phase III trial was being prepared for publication.
The Panel noted that Samsca was first approved on 5 May 2009. This meant that the 2008 Code applied and the Joint Position 2005. One trial with UK involvement completed in July 2006 and Otsuka submitted it was published in JAMA in March 2007. The study had been published within one year of Samsca being approved and commercially available as required. The Panel ruled no breach of the 2008 Code including Clause 2.
The Panel noted Otsuka’s submission that seven other trials were either Phase 1 trials on healthy volunteers and/or used a different formulation of tolvaptan to that licensed. The Panel noted that the Joint Position 2005 did not require disclosure of exploratory trials unless they were of significant medical importance and might have an impact on the marketed product’s labelling. The Panel was unsure whether the results were of significant medical importance. The complainant had not provided any details in this regard. The Panel considered publication of such data was preferable, however on the information before it there appeared to be no need to disclose the results of the trials under the 2008 Code. The Panel ruled no breach of the 2008 Code including Clause 2.
An anonymous, contactable member of the public complained about the information published as ‘Clinical Trial Transparency: an assessment of the disclosure results of company-sponsored trials associated with new medicines approved recently in Europe’. The study was published in Current Medical Research & Opinion (CMRO) on 11 November 2013.
The study authors were Dr B Rawal, Research, Medical and Innovation Director at the ABPI and B R Deane, a freelance consultant in pharmaceutical marketing and communications. Publication support for the study was funded by the ABPI.
The study surveyed various publicly available information sources for clinical trial registration and disclosure of results searched from 27 December 2012 to 31 January 2013. It covered 53 new medicines (except vaccines and fixed dose combinations) approved for marketing by 34 companies by the European Medicines Agency (EMA) in 2009, 2010 and 2011. It included all completed company-sponsored clinical trials conducted in patients and recorded on a clinical trial registry and/or included in a European Public Assessment Report (EPAR). The CMRO publication did not include the specific data for each product. This was available via a website link and was referred to by the complainant. The study did not aim to assess the content of disclosure against any specific requirements.
COMPLAINT
The complainant stated that the study detailed a number of companies which had not disclosed their clinical trial results in line with the ABPI for licensed products. The complainant provided a link to relevant information which included the published study plus detailed information for each product that was assessed:
The explanation of terms given in the documentation was as follows:
Total
Total number of trials identified which were completed and/or with results disclosed
Unevaluable
Trials within the total which could not be evaluated (due to either trial completion date or publication date being missing or unclear) – excluded from the analysis
Evaluable
Trials with all criteria present including dates, and hence the base which could be evaluated for the assessment
Results disclosed in timeframe
Evaluable trials which fully complied with publication requirements, ie summary results disclosed (in registry or journal) within 12 months of either first regulatory approval date or trial completion date, whichever was later
Disclosure percentage
Proportion of evaluable trials which were fully disclosed
Completed before end of January 2012
Number of studies completed before end January 2012 (or already disclosed)
Results disclosed at all
Number of trials with any publication of results at any time
Disclosure percentage at 31 January 2013
Proportion of trials completed by end January 2012 which were now disclosed
* * *
The complainant alleged that all of the companies listed had breached Clauses 2, 9 and 21 of the Code.
When writing to Otsuka, the Authority drew attention to Clauses 1.8 and 21.3 of the Second 2012 Edition of the Code and noted that previous versions of the Code might also be relevant.
RESPONSE
In its initial response Otsuka UK stated that as the UK affiliate of an international pharmaceutical company it had no clinical research department, did not sponsor any good clinical practice (GCP)studies and did not fund any investigator-initiated studies for any of its products including Samsca. All Otsuka sponsored clinical trials were organised, funded and managed by the global organisation which was not located in the UK. Otsuka stated that Samsca was first authorised in the US on 5 May 2009 and in the EU on 3 August 2009. It was currently authorised in 40 countries and marketed in 14.
The case preparation manager asked Otsuka UK to provide further information.
Otsuka summarised the tolvaptan clinical trials and provided what it described as an exhaustive, confidential, list from the tolvaptan investigators’ brochure. Of the trials listed only one had a UK nexus. This trial was in heart failure with a clinicaltrial.gov identifier, NCT00071331. A link was provided to the registry entry.
A printout of the entry was provided. There was a link at the bottom of the results page to the main results publication (Konstam et al March 2007). This paper was available on the Journal of the American Medical Association (JAMA) website free of charge
The summary output for each medicine set out the sources for all trials found, irrespective of sponsor and an analysis of publication disclosure in the form of a table which gave details for the studies for each product. The data for Samsca (tolvaptan) were as follows:
by following the JAMA link from the results page. Otsuka submitted that this therefore fulfilled the requirements laid out in the Joint Position.
Given that the results were published within a year of completing the study and linked to the registry entry and that the results publication from that link was available free of charge Otsuka submitted that there had been no breach of Clause 21. Equally, as Otsuka had fulfilled its requirements in this regard there was no breach of Clause 1.8. Clearly as there was no breach of other clauses there was no breach of Clauses 9.1 and 2.
In response to a request for further information Otsuka confirmed that three multicentre, international trials identified by the Panel from the list provided by Otsuka did not involve UK sites.
With regard to the Panel’s query about seven studies on the list provided by Otsuka, the company submitted that these studies were conducted before the European database EudraCT was established. Otsuka submitted that the Joint Position on the
Disclosure of Clinical Trial Information via Clinical Trial Registries and Databases (2005 & 2009) and the Joint Position on the Publication of Clinical Trial Results in the Scientific Literature (2010) were established subsequent to these dates and did not mandate retroactive publication of studies. Four of the studies were Phase 1 studies in healthy controls and at that time did not require reporting. Moreover they were with the spray-dried formulation and hence not the approved formulation. Two studies were Phase 1 studies in healthy subjects. One study was also Phase 1 with tolvaptan sachets which was not an approved formulation.
GENERAL COMMENTS FROM THE PANEL
The Panel noted the ABPI involvement in the study. However, a complaint had been received and it needed to be considered in the usual way in line with the PMCPA Constitution and Procedure. The Panel noted that all the cases would be considered under the Constitution and Procedure in the Second 2012 Edition as this was in operation when the complaint was received. The addendum (1 July 2013 which came into effect on 1 November 2013) to this Code only related to Clause 16 and was not relevant to the consideration of these cases.
The Panel noted that the study concluded that the results of over three quarters of all company sponsored clinical trials were disclosed within a year of completion or regulatory approval and almost 90% were disclosed by 31 January 2013 which suggested transparency was now better than had sometimes been reported previously.
The Panel considered that the first issue to be determined was whether the matter was covered by the ABPI Code. If the research was conducted on behalf of a UK pharmaceutical company (whether directly or via a third party) then it would be covered by the ABPI Code. If a study was run by a non UK company but had UK involvement such as centres, investigators, patients etc it was likely that the Code would apply. The Panel appreciated the global nature of much pharmaceutical company sponsored clinical research and a company located in the UK might not be involved in research that came within the ABPI Code. It was a well established principle that UK pharmaceutical companies were responsible for the activities of overseas affiliates if such activities related to UK health professionals or were carried out in the UK.
Clause 21.3 of the Second 2012 Edition of the Code stated that companies must disclose details of clinical trials in accordance with the Joint Position on the Disclosure of Clinical Trial Information via Clinical Trial Registries and Databases and the Joint Position on the Publication of Clinical Trial Results in the Scientific Literature.
The relevant supplementary information stated that this clause required the provision of details about ongoing clinical trials (which must be registered within 21 days of initiation of patients enrolment) and completed trials for medicines licensed for use in at least one country. Further information was to be found in the Joint Position on the Disclosure of Clinical Trial Information via Clinical Trial Registries and Databases 2009 and the Joint Position on the Publication of Clinical Trial Results in the Scientific Literature 2010, both at http://clinicaltrials.ifpma.org.
The Panel noted that the first Joint Position on the Disclosure of Clinical Trial Information via Clinical Trial Registries and Databases was agreed in 2005 by the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA), the European Federation of Pharmaceutical Industries and Associations (EFPIA), the Japanese Pharmaceutical Manufacturers Association (JPMA) and the Pharmaceutical Research and Manufacturers of America (PhRMA). The announcement was dated 6 January 2005.
The Panel noted that Article 9, Clinical Research and Transparency, of the most recent update of the IFPMA Code of Practice (which came into operation on 1 September 2012) included a statement that companies disclose clinical trial information as set out in the Joint Position on the Disclosure of Clinical Trial Information via Clinical Trial Registries and Databases (2009) and the Joint Position on the Publication of Clinical Trial Results in the Scientific Literature (2010). As companies had, in effect, agreed the joint positions their inclusion in the IFPMA Code should not have made a difference in practice to IFPMA member companies but meant that IFPMA member associations had to amend their codes to reflect Article 9. The Second 2012 Edition of the ABPI Code fully reflected the requirements of the IFPMA Code. The changes introduced in the ABPI Code were to update the date of the Joint Position on the Disclosure of Clinical Trial Information and to include the new requirement to disclose in accordance with the Joint Position on the Publication of Clinical Trial Results. Pharmaceutical companies that were members of national associations but not of IFPMA would have additional disclosure obligations once the national association amended its code to meet IFPMA requirements. The disclosures set out in the joint positions were not required by the EFPIA Codes.
The Panel noted that even if the UK Code did not apply many of the companies listed by the complainant were members of IFPMA and/or EFPIA.
The Panel considered that it was good practice for clinical trial results to be disclosed for medicines which were first approved and commercially available after 6 January 2005 (the date of the first joint position). This was not necessarily a requirement of the ABPI Codes from that date as set out below.
As far as the ABPI Code was concerned, the Panel noted that the first relevant mention of the Joint Position on the Disclosure of Clinical Trial Information via Clinical Trial Registries and Databases 2005 was in the supplementary information to Clause 7.5 of the 2006 Code:
‘Clause 7.5 Data from Clinical Trials
Companies must provide substantiation following a request for it, as set out in Clause 7.5. In addition, when data from clinical trials is used companies must ensure that where necessary that data has been registered in accordance with the Joint Position on the Disclosure of Clinical Trial Information via Clinical Trial Registries and Databases 2005.’
Clause 7.5 of the 2006 Code required that substantiation be provided at the request of health professionals or appropriate administrative staff. Substantiation of the validity of indications approved in the marketing authorization was not required. The Panel considered this was not relevant to the complaint being considered which was about disclosure of clinical trial results. The Joint Position 2005 was mentioned in the supplementary information to Clause 21.5 but this did not relate to any Code requirement to disclose clinical trial results.
In the 2008 ABPI Code (which superceded the 2006 Code and came into operation on 1 July 2008 with a transition period until 31 October 2008 for newly introduced requirements), Clause 21 referred to scientific services and Clause 21.3 stated:
‘Companies must disclose details of clinical trials.’
The relevant supplementary information stated:
‘Clause 21.3 Details of Clinical Trials
This clause requires the provision of details about ongoing clinical trials (which must be registered within 21 days of initiation of patients enrolment) and completed trials for medicines licensed for use in at least one country. Further information can be found in the Joint Position on the Disclosure of Clinical Trial Information via Clinical Trial Registries and Databases 2005 (http:// clinicaltrials.ifpma.org).
Details about clinical trials must be limited to factual and non-promotional information. Such information must not constitute promotion to health professionals, appropriate administrative staff or the public.’
In the 2011 Code (which superceded the 2008 Code and came into operation on 1 January 2011 with a transition period until 30 April 2011 for newly introduced requirements), the supplementary information to Clause 21.3 was updated to refer to the 2008 IFPMA Joint Position.
In the Second 2012 Edition (which came into operation on 1 July 2012 with a transition period until 31 October 2012 for newly introduced requirements), changes were made to update the references to the joint position and to include the Joint Position on the Publication of Clinical Trial Results in the Scientific Literature. Clause 21.3 now stated:
‘Companies must disclose details of clinical trials in accordance with the Joint Position on the Disclosure of Clinical Trial Information via Clinical
Trial Registries and Databases and the Joint Position on the Publication of Clinical Trial Results in the Scientific Literature.’
The relevant supplementary information stated:
‘Clause 21.3 Details of Clinical Trials
This clause requires the provision of details about ongoing clinical trials (which must be registered within 21 days of initiation of patients enrolment) and completed trials for medicines licensed for use in at least one country. Further information can be found in the Joint Position on the Disclosure of Clinical Trial Information via Clinical Trial Registries and Databases 2009 and the Joint Position on the Publication of Clinical Trial Results in the Scientific Literature 2010, both at http:// clinicaltrials.ifpma.org.
Details about clinical trials must be limited to factual and non-promotional information. Such information must not constitute promotion to health professionals, appropriate administrative staff or the public.’
The Panel noted that in the 2014 ABPI Code the disclosure requirements which had previously been stated in Clause 21 had been moved to Clause 13. In addition, the supplementary information stated that companies must include on their website information as to where details of their clinical trials could be found. The 2014 Code would come into effect on 1 May 2014 for newly introduced requirements following a transition period from 1 January 2014 until 30 April 2014.
The Panel examined the Joint Position on the Disclosure of Clinical Trial Information which was updated on 10 November 2009 and superseded the Joint Position 2008. With regard to clinical trial registries the document stated that all trials involving human subjects for Phase I and beyond at a minimum should be listed. The details should be posted no later than 21 days after the initiation of enrolment. The details should be posted on a free publicly accessible internet-based registry. Examples were given. Each trial should be given a unique identifier to assist in tracking. The Joint Position 2009 provided a list of information that should be provided and referred to the minimum Trial Registration Data Set published by the World Health Organisation (WHO). The Joint Position 2009 referred to possible competitive sensitivity in relation to certain data elements and that, in exceptional circumstances, this could delay disclosure at the latest until after the medicinal product was first approved in any country for the indication being studied. Examples were given.
The Panel noted that the complaint related to the disclosure of clinical trial results.
With regard to the disclosure of clinical trial results the Joint Position 2009 stated that the results for a medicine that had been approved for marketing and was commercially available in at least one country should be publicly disclosed. The results should be posted no later than one year after the medicine was first approved and commercially available. The results for trials completed after approval should be posted one year after trial completion – an adjustment to this schedule was possible to comply with national laws or regulations or to avoid compromising publication in a peer-reviewed medical journal.
The Joint Position 2009 included a section on implementation dates and the need for companies to establish a verification process.
The Joint Position 2005 stated that the results should be disclosed of all clinical trials other than exploratory trials conducted on a medicine that was approved for marketing and was commercially available in at least one country. The results generally should be posted within one year after the medicine was first approved and commercially available unless such posting would compromise publication in a peer-reviewed medical journal or contravene national laws or regulations. The Joint Position 2008 was dated 18 November 2008 and stated that it superseded the Joint Position 2005 (6 January and 5 September). The Joint Position 2008 stated that results should be posted no later than one year after the product was first approved and commercially available in any country. For trials completed after initial approval these results should be posted no later than one year after trial completion. These schedules would be subject to adjustment to comply with national laws or regulations or to avoid compromising publication in a peer reviewed medical journal.
The Joint Position on the Publication of Clinical Trial Results in the Scientific Literature was announced on 10 June 2010. It stated that all industry sponsored clinical trials should be considered for publication and at a minimum results from all Phase III clinical trials and any clinical trials results of significant medical importance should be submitted for publication. The results of completed trials should be submitted for publication wherever possible within 12 months and no later than 18 months of the completion of clinical trials for already marketed medicines and in the case of investigational medicines the regulatory approval of the new medicine or the decision to discontinue development.
Having examined the various codes and joint positions, the Panel noted that the Joint Position 2005 excluded any clinical trials completed before 6 January 2005. The position changed on 18 November 2008 as the Joint Position 2008 did not have any exclusion relating solely to the date the trial completed. The Joint Position 2009 was similar to the Joint Position 2008 in this regard.
The Panel noted that deciding which Code applied, and thus which joint position, was complicated. It noted that the 2011 Code which, taking account the transition period, came into operation on 1 May 2011 was the first edition of the Code to refer to the Joint Position 2008.
The Panel concluded that from 1 November 2008, (allowing for the transition period) until 30 April 2011 under the 2008 Code companies were required to follow the Joint Position 2005. From 1 May 2011 until 31 October 2012 under the 2012 Code companies were required to follow the Joint Position 2008. Since 1 November 2012 companies were required to follow the Joint Position 2009. The Panel considered that since the 2008 Code companies were, in effect, required to comply with the Joint Position cited in the relevant supplementary information. The relevant supplementary information gave details of what was meant by Clause 21.3 (Clause 13.1 in the 2014 Code). The
Panel accepted that the position was clearer in the Second 2012 Edition of the Code. The Panel noted that the 2011 Code should have been updated to refer to the Joint Position 2009.
For medicines first licensed and commercially available in any country from 1 November 2008 until 30 April 2011 the results of clinical trials completed before 6 January 2005 would not have to be posted.
From 1 May 2011 there was no exclusion of trials based solely on completion date and so for a product first licensed and commercially available anywhere in the world after 1 May 2011 the applicable joint positions required relevant clinical trial results to be posted within a year of the product being first approved and commercially available or within a year of trial completion for trials completed after the medicine was first available.
Noting that the complaint concerned licensed products the Panel considered that the trigger for disclosure was the date the product was first approved and commercially available anywhere in the world. This would determine which version of the Code (and joint position) applied for trials completed prior to first approval. The next consideration was whether the trial completed before or after this date. For trials completing after the date of first approval, the completion date of the trial would determine which Code applied. The Panel considered that the joint positions encouraged disclosure as soon as possible and by no later than 1 year after first availability or trial completion as explained above. The Panel thus considered that its approach was a fair one. In this regard, it noted that the complaint was about whether or not trial results had been disclosed, all the joint positions referred to disclosure within a one year timeframe and companies needed time to prepare for disclosure of results. The Panel considered that the position concerning unlicensed indications or presentations of otherwise licensed medicines etc would have to be considered on a case by case basis bearing in mind the requirements of the relevant joint position and the legitimate need for companies to protect intellectual property rights. The Panel followed the decision tree set out below which it considered set out all the relevant possibilities.
Decision Tree - Developed by the Panel when considering the complaint about the disclosure of clinical trial results - for expanded chart see Case Report PDF

During its development of the decision tree, the Panel sought advice from Paul Woods, BPharm MA (Medical Ethics and Law) of Paul Woods Compliance Ltd who provided an opinion. Mr Woods was not provided with details of the complaint or any of the responses. The advice sought was only in relation to the codes and joint positions.
The Panel considered the complaint could be read in two ways: firstly that the companies listed had not disclosed the data referred to in the CMRO publication relating to the products named or secondly, more broadly, that the companies had not disclosed the clinical trial data for the product named ie there could be studies in addition to those looked at in the CMRO publication. The Panel decided that it would consider these cases in relation to the studies covered by the CMRO publication and not on the broader interpretation. Companies would be well advised to ensure that all the clinical trial results were disclosed as required by the Codes and joint positions. The Panel considered that there was no complaint about whether the results disclosed met the requirements of the joint positions so this was not considered. In the Panel’s view the complaint was only about whether or not study results had been disclosed and the timeframe for such disclosure.
The CMRO publication stated that as far as the IFPMA Joint Position was concerned implementation had been somewhat variable in terms of completeness and timing. The Panel noted that a number of studies were referred to in the CMRO publication as ‘unevaluable’ and these were not specifically mentioned by the complainant. The CMRO publication focussed on the disclosure of evaluable trial results and the Panel only considered those evaluable trials.
The Panel noted that its consideration of these cases relied upon the information provided by the respondent companies. The CMRO publication did not identify the studies evaluated; it only provided quantitative data. The Panel noted that the study ran from 27 December 2012 to 31 January 2013 and was published in November 2013. The Panel considered that companies that might not have been in line with various disclosure requirements had had a significant period of time after the study completed and prior to the current complaint being received to have disclosed any missing information. It appeared that the authors of the CMRO publication had contacted various companies for additional information.
The Panel noted that the case preparation manager raised Clause 1.8 of the Second 2012 Edition with the companies. The supplementary information to Clause 1.8, Applicability of Codes, inter alia, referred to the situation when activities involved more than one country or where a pharmaceutical company based in one country was involved in activities in another country. The complainant had not cited Clause 1.8. The Panel noted that any company in breach of any applicable codes, laws or regulations would defacto also be in breach of Clause 1.8 of the Code; the converse was true. The Panel thus decided that as far as this complaint was concerned, any consideration of a breach or otherwise of Clause 1.8 was covered by other rulings and it decided, therefore, not to make any ruling regarding this clause (or its equivalent in earlier versions of the Code).
PANEL RULING IN CASE AUTH/2670/11/13
The Panel noted the CMRO publication in that 18 evaluable studies had not been disclosed within the timeframe. The disclosure percentage was 36%. Ten studies completed before the end of 2012 had not been disclosed. The disclosure percentage at 31 January 2013 of trials completed before the end of January 2012 was 64%. A footnote stated that the undisclosed Phase I/II trials comprised of trials completed before reporting requirements. Trials with no US IND therefore not subject to FDAAA 801 requirements. The undisclosed Phase III trial was being prepared for publication.
The Panel noted that Samsca was first approved on 5 May 2009. This meant that the 2008 Code applied and the Joint Position 2005. One trial with UK involvement completed in July 2006. Otsuka submitted it was published in JAMA in March 2007 and so in this regard the study had been published within one year of Samsca being approved and commercially available as required. The Panel ruled no breach of Clause 21.3 of the 2008 Code and consequently no breach of Clauses 9.1 and 2.
The Panel noted Otsuka’s submission that another three trials queried by the Panel had no UK involvement. The Panel did not know whether the results of these trials had been disclosed. However as there was no UK involvement the Panel considered the matter did not come within the scope of the UK Code and therefore ruled no breach.
The Panel noted Otsuka’s submission that seven other trials were either Phase 1 trials on healthy volunteers and/or used a different formulation of tolvaptan to that licensed. The Panel noted that the Joint Position 2005 did not require disclosure of exploratory trials unless they were of significant medical importance and might have an impact on marketed product’s labelling. The Panel was unsure whether the results were of significant medical importance. The complainant had not provided any details in this regard. The Panel considered publication of such data was preferable, however on the information before it there appeared to be no need to disclose the results of the trials under the 2008 Code. The Panel ruled no breach of Clause 21.3 of the 2008 Code and consequently no breach of Clauses 9.1 and 2.
The Panel noted that none of the additional seven trials had any UK involvement and the Panel considered the matter did not come within the scope of the Code and therefore ruled no breach.
Complaint received 21 November 2013
Case completed 20 March 2014
see cases: 3005,2908,2906,2903,2898,2763,2676,2674,2673,2672,2671,2670,2669,2667,2666,2665,2664,
2663,2662,2661,2659,2657,2654


