Case Summary
An anonymous, non-contactable complainant complained about a journal advertisement for Rienso (ferumoxytol), issued by Takeda, and the website (www.anaemiazone.co.uk) referred to within it. Rienso was indicated for the intravenous (IV) treatment of iron deficiency anaemia in adults with chronic kidney disease (CKD). Patients were treated with one or two IV doses of 510mg depending on their pre-treatment status. Rienso particles consisted of a bioactive iron oxide core protected by a polyglucose sorbitolcarboxymethylether (PSC) coating.
The complainant noted that the advertisement described Rienso as 'high dose' but did not state what this was in comparison to. The complainant stated that Monofer (iron (III) isomaltoside 1000) could be given at doses of 500mg in haemodialysis patients, and 20mg/kg otherwise, Rienso appeared to be low dose.
The detailed response from Takeda is given below.
The Panel noted that Takeda submitted that 'high dose' was used in conjunction with 'Short course' and 'Rapid bolus injection' to describe the attributes of Rienso's administration and was not used comparatively. The Panel did not consider that the use of 'high dose' in this context was a hanging comparison. No breach of the Code was ruled. With regard to the complainant's further allegation that, compared with Monofer, Rienso appeared to be low dose, the Panel noted that literature provided by Takeda described high dose iron as doses greater than 200mg in a one month period. In the Panel's view the description of Rienso as high dose was supported by the literature. The Panel ruled no breach of the Code.
The complainant noted that the website described Rienso was described as a new IV iron whereas it had been available for over a year. The website stated that both the structure was designed to allow rapid administration of high doses. The complainant alleged that this was unlikely because Rienso appeared to cause more side effects than other IV irons especially immunological reactions and that the high dose was also incorrect. Section 3 stated that Monofer took five injections for 1g whereas it only took two. Section 3 further stated that all IV irons were contraindicated in hypersensitivity to Rienso or other iron preparations. The complainant alleged that this was only true for Rienso. The complainant further alleged that the cost effectiveness section was misleading and unfair because it only took into account the cost of the medicine and not the administration cost. The complainant alleged that the claim that Rienso was convenient was debatable in non haemodialysis patients as three other preparations only requiredone infusion. Finally, the cost-competitive statement was repeated although only the medicine cost was referred to.
The Panel noted that the Rienso SPC listed 15 June 2012 as the date of first authorization. The Panel further noted Takeda's account of its activities subsequent to that date and its submission that Rienso could not have been promoted before 8 August 2012 as this was when product training was completed. The Panel noted, however, that a contract between an agency and Takeda stated that '[the agency] would carry out and perform the services…' with effect from the commencement date….' ie from 23 July. The services included navigating the changing NHS in the correct timelines with the correct information (advanced product notification (APN) and budget impact model) to ensure appropriate local product update. Reference was made to engaging the right decision makers in a local health economy and key opinion leader advocacy at launch. The advanced product notification referred to budgetary conversations that would take place with relevant NHS budget holders from 23 July but given that this was 5-6 weeks after Rienso had received its marketing authorization, the Panel considered that such activity was promotional. In that regard Rienso had thus been promoted since 23 July 2012 and so could not be described as 'new' beyond 22 July 2013. The Panel however, that the product had been described as new on the website until 1 August 2013. A breach of the Code was ruled.
The Panel noted the complainant's view that the site stated the structure was designed to allow rapid administration of high dose but that seemed unlikely since Rienso appeared to cause more side effects than other IV irons (especially immunological reactions). The Panel understood the complainant to mean that as Rienso caused more side effects than other IV irons (especially immunological reactions) it was unlikely that the structure was designed to allow rapid administration of a high dose. The complainant did not provide any evidence to support this allegation. The Panel noted that the website stated that 'The unique structure of Rienso is designed to allow rapid administration of high doses (510mg) of iron'. A bullet point below stated that the protective PSC coating acted as a shield to reduce immunological sensitivity and release of free iron. The Panel noted that the Rienso SPC stated that in clinical trials, serious hypersensitivity or hypotensive reactions to Rienso were uncommon (reported in 3 (0.2%) of patients with CKD). The Panel further noted that all of the IV iron SPCs provided by Takeda stated that parenteral administration of all iron complexes might cause immediate severe and potentially lethal hypersensitivity reactions. In the Panel's view noevidence was provided to support the allegation that Rienso caused more side effects than other IV irons (especially immunological reactions). The Panel noted that according to the SPC, Rienso was administered as an undiluted IV injection delivered at a rate of up to 1ml/sec (30mg/sec) ie at least 17 seconds per vial. Provenzano et al stated that in vitro data suggested that ferumoxytol contained less free iron than other IV preparations and it was perhaps these physicochemical characteristics that permitted the rapid administration of larger doses compared with currently available iron preparations. The Panel considered that the statement 'The unique structure of Rienso is designed to allow rapid administration of high doses (510mg) of iron' was accurate, reflected the evidence and was capable of substantiation. The Panel thus ruled no breach of the Code.
The Panel noted its comments above and considered that its ruling of no breach of the Code in relation to describing Rienso as 'high dose' also applied to the website.
The Panel noted that one section of the website showed that to deliver 1g of iron required 2 bolus injections of Rienso and 5 bolus injections of Monofer. The Panel noted Takeda's submission that when the website was certified, Monofer injection could only be administered in maximum doses of 200mg in patients on haemodialysis but that the SPC had since been amended to allow a maximum dose of 500mg in patients on haemodialysis. The updated Monofer SPC was uploaded onto the eMC on 17 July 2013, 13 days before the complaint was submitted. Takeda had missed the update as it only monitored the eMC once a month; the company had acknowledged that the website had thus included outdated information about Monofer for some days. The material at issue could not be substantiated. Breaches of the Code were ruled.
The Panel noted the website stated that 'As with all IV irons, the use of Rienso is contraindicated in cases of: hypersensitivity to Rienso, its excipients or other iron preparations'; the complainant alleged that this was only true for Rienso. The Panel noted following a comparison of its competitors' SPCs, Rienso appeared to be the only one with hypersensitivity to other iron preparations listed as an explicit contraindication. The Panel considered that the claim thus did not reflect the available evidence and was not capable of substantiation. Breaches of the Code were ruled.
The Panel noted the allegation that the cost effectiveness section of the website only took into account the cost of the medicine and not the true cost to administer and was therefore misleading and an unfair comparison. The Panel noted Takeda's submission that while cost effectiveness was used to indicate the section of the website where cost was presented, Takeda had only claimed that Rienso was a cost-competitive option for rapid and convenient IV iron management. In the Panel's view, use of the heading 'cost effectiveness' to describe a section of the website which only detailed acquisition cost was misleading. The tableof data provided listed the 'Calculated NHS list price to administer 1g of IV iron' and thus it would be clear to the reader that the costs of the five medicines cited were acquisition costs only and did not take into account any related administration costs. Nonetheless, the Panel considered that to put such data under a heading of 'cost effectiveness' was misleading and breaches of the Code were ruled.
The Panel noted the allegation that the convenience of Rienso was debatable. It further noted Takeda's submission that Rienso offered a convenient option to patients as well as health professionals as it allowed 1g of iron to be administered with two injections in a short course, high dose, rapid bolus injection administered in 17 seconds with 30 minutes of post-dose observation over two to eight days. On balance the Panel considered that in light of current IV iron therapy, the claim that Rienso was convenient was not misleading. In that regard, the Panel ruled no breach of the Code.
The Panel noted its rulings above and considered that high standards had not been maintained. A breach of the Code was ruled. The Panel did not consider, however, that the material at issue was such as to bring discredit upon, or reduce confidence in, the pharmaceutical industry. No breach of Clause 2 was ruled.
CASE AUTH/2623/7/13 ANONYMOUS v TAKEDA
Promotion of Rienso
An anonymous, non-contactable complainant complained about a journal advertisement for Rienso (ferumoxytol), issued by Takeda, and the website (www.anaemiazone.co.uk) referred to within it. Rienso was indicated for the intravenous (IV) treatment of iron deficiency anaemia in adults with chronic kidney disease (CKD). Patients were treated with one or two IV doses of 510mg depending on their pre-treatment status. Rienso particles consisted of a bioactive iron oxide core protected by a polyglucose sorbitolcarboxymethylether (PSC) coating.
The complainant noted that the advertisement described Rienso as ‘high dose’ but did not state what this was in comparison to. The complainant stated that Monofer (iron (III) isomaltoside 1000) could be given at doses of 500mg in haemodialysis patients, and 20mg/kg otherwise, Rienso appeared to be low dose.
The detailed response from Takeda is given below.
The Panel noted that Takeda submitted that ‘high dose’ was used in conjunction with ‘Short course’ and ‘Rapid bolus injection’ to describe the attributes of Rienso’s administration and was not used comparatively. The Panel did not consider that the use of ‘high dose’ in this context was a hanging comparison. No breach of the Code was ruled. With regard to the complainant’s further allegation that, compared with Monofer, Rienso appeared to be low dose, the Panel noted that literature provided by Takeda described high dose iron as doses greater than 200mg in a one month period. In the Panel’s view the description of Rienso as high dose was supported by the literature. The Panel ruled no breach of the Code.
The complainant noted that the website described Rienso was described as a new IV iron whereas it had been available for over a year. The website stated that both the structure was designed to allow rapid administration of high doses. The complainant alleged that this was unlikely because Rienso appeared to cause more side effects than other IV irons especially immunological reactions and that the high dose was also incorrect. Section 3 stated that Monofer took five injections for 1g whereas it only took two. Section 3 further stated that all IV irons were contraindicated in hypersensitivity to Rienso or other iron preparations. The complainant alleged that this was only true for Rienso. The complainant further alleged that the cost effectiveness section was misleading and unfair because it only took into account the cost of the medicine and not the administration cost. The complainant alleged that the claim that Rienso was convenient was debatable in non haemodialysis patients as three other preparations only required one infusion. Finally, the cost-competitive statement was repeated although only the medicine cost was referred to.
The Panel noted that the Rienso SPC listed 15 June 2012 as the date of first authorization. The Panel further noted Takeda’s account of its activities subsequent to that date and its submission that Rienso could not have been promoted before 8 August 2012 as this was when product training was completed. The Panel noted, however, that a contract between an agency and Takeda stated that ‘[the agency] would carry out and perform the services…’ with effect from the commencement date….’ ie from 23 July. The services included navigating the changing NHS in the correct timelines with the correct information (advanced product notification (APN) and budget impact model) to ensure appropriate local product update. Reference was made to engaging the right decision makers in a local health economy and key opinion leader advocacy at launch. The advanced product notification referred to budgetary conversations that would take place with relevant NHS budget holders from 23 July but given that this was 5-6 weeks after Rienso had received its marketing authorization, the Panel considered that such activity was promotional. In that regard Rienso had thus been promoted since 23 July 2012 and so could not be described as ‘new’ beyond 22 July 2013. The Panel however, that the product had been described as new on the website until 1 August 2013. A breach of the Code was ruled.
The Panel noted the complainant’s view that the site stated the structure was designed to allow rapid administration of high dose but that seemed unlikely since Rienso appeared to cause more side effects than other IV irons (especially immunological reactions). The Panel understood the complainant to mean that as Rienso caused more side effects than other IV irons (especially immunological reactions) it was unlikely that the structure was designed to allow rapid administration of a high dose. The complainant did not provide any evidence to support this allegation. The Panel noted that the website stated that ‘The unique structure of Rienso is designed to allow rapid administration of high doses (510mg) of iron’. A bullet point below stated that the protective PSC coating acted as a shield to reduce immunological sensitivity and release of free iron. The Panel noted that the Rienso SPC stated that in clinical trials, serious hypersensitivity or hypotensive reactions to Rienso were uncommon (reported in 3 (0.2%) of patients with CKD). The Panel further noted that all of the IV iron SPCs provided by Takeda stated that parenteral administration of all iron complexes might cause immediate severe and potentially lethal hypersensitivity reactions. In the Panel’s view no evidence was provided to support the allegation that Rienso caused more side effects than other IV irons (especially immunological reactions). The Panel noted that according to the SPC, Rienso was administered as an undiluted IV injection delivered at a rate of up to 1ml/sec (30mg/sec) ie at least 17 seconds per vial. Provenzano et al stated that in vitro data suggested that ferumoxytol contained less free iron than other IV preparations and it was perhaps these physicochemical characteristics that permitted the rapid administration of larger doses compared with currently available iron preparations. The Panel considered that the statement ‘The unique structure of Rienso is designed to allow rapid administration of high doses (510mg) of iron’ was accurate, reflected the evidence and was capable of substantiation. The Panel thus ruled no breach of the Code.
The Panel noted its comments above and considered that its ruling of no breach of the Code in relation to describing Rienso as ‘high dose’ also applied to the website.
The Panel noted that one section of the website showed that to deliver 1g of iron required 2 bolus injections of Rienso and 5 bolus injections of Monofer. The Panel noted Takeda’s submission that when the website was certified, Monofer injection could only be administered in maximum doses of 200mg in patients on haemodialysis but that the SPC had since been amended to allow a maximum dose of 500mg in patients on haemodialysis. The updated Monofer SPC was uploaded onto the eMC on 17 July 2013, 13 days before the complaint was submitted. Takeda had missed the update as it only monitored the eMC once a month; the company had acknowledged that the website had thus included outdated information about Monofer for some days.
The material at issue could not be substantiated. Breaches of the Code were ruled.
The Panel noted the website stated that ‘As with all IV irons, the use of Rienso is contraindicated in cases of: hypersensitivity to Rienso, its excipients or other iron preparations’; the complainant alleged that this was only true for Rienso. The Panel noted following a comparison of its competitors’ SPCs, Rienso appeared to be the only one with hypersensitivity to other iron preparations listed as an explicit contraindication. The Panel considered that the claim thus did not reflect the available evidence and was not capable of substantiation. Breaches of the Code were ruled.
The Panel noted the allegation that the cost effectiveness section of the website only took into account the cost of the medicine and not the true cost to administer and was therefore misleading and an unfair comparison. The Panel noted Takeda’s submission that while cost effectiveness was used to indicate the section of the website where cost was presented, Takeda had only claimed that Rienso was a cost-competitive option for rapid and convenient IV iron management. In the Panel’s view, use of the heading ‘cost effectiveness’ to describe a section of the website which only detailed acquisition cost was misleading. The table of data provided listed the ‘Calculated NHS list price to administer 1g of IV iron’ and thus it would be clear to the reader that the costs of the five medicines cited were acquisition costs only and did not take into account any related administration costs. Nonetheless, the Panel considered that to put such data under a heading of ‘cost effectiveness’ was misleading and breaches of the Code were ruled.
The Panel noted the allegation that the convenience of Rienso was debatable. It further noted Takeda’s submission that Rienso offered a convenient option to patients as well as health professionals as it allowed 1g of iron to be administered with two injections in a short course, high dose, rapid bolus injection administered in 17 seconds with 30 minutes of post-dose observation over two to eight days. On balance the Panel considered that in light of current IV iron therapy, the claim that Rienso was convenient was not misleading. In that regard, the Panel ruled no breach of the Code.
The Panel noted its rulings above and considered that high standards had not been maintained. A breach of the Code was ruled. The Panel did not consider, however, that the material at issue was such as to bring discredit upon, or reduce confidence in, the pharmaceutical industry. No breach of Clause 2 was ruled.
An anonymous, non-contactable complainant complained about a Rienso (ferumoxytol) advertisement (ref April 2013 UK/RIE/1304/0040) issued by Takeda UK Ltd published in the Journal of Renal Nursing, 4 July 2013 and the website (www. anaemiazone.co.uk) (ref FE120916) referred to within it. Rienso was indicated for the intravenous (IV) treatment of iron deficiency anaemia in adults with chronic kidney disease (CKD). Patients were treated with one or two IV doses of 510mg depending on their pre-treatment haemoglobin level and body weight. Rienso particles consisted of a bioactive iron oxide core protected by a polyglucose sorbitolcarboxymethylether (PSC) coating.
A JOURNAL ADVERTISEMENT
COMPLAINT
The complainant noted that the advertisement described Rienso as ‘high dose’ but did not state what this was in comparison to. The complainant stated that he/she had been informed that Monofer (iron (III) isomaltoside 1000) could be given at doses of 500mg in haemodialysis patients, and 20mg/kg otherwise and in that context Rienso appeared to be low dose.
When writing to Takeda, the Authority asked it to respond in relation to Clause 7.2.
RESPONSE
Takeda noted that the complainant asked for consideration of the term ‘high dose’ and queried what it was being compared to. With specific reference to Clause 7.2, the complainant appeared to be describing the term as a hanging comparison. Takeda submitted that according to the Code such comparisons whereby a medicine was described as being better or stronger or suchlike without stating that with which it was being compared were hanging comparisons and were not allowed. Takeda did not consider that the term ‘high dose’ compared Rienso to other medicines and submitted that it described one of Rienso’s attributes. ‘High dose’ was presented in the advertisement on the middle of three lines of the same font size and colour indicating that the three lines were to be read in conjunction with each other as one statement thus describing Rienso as a ‘Short course, High dose, Rapid bolus injection’. This summary statement described the attributes of Rienso’s administration and was not inconsistent with the summary of product characteristics (SPC). Takeda submitted that its decision to describe Rienso as high dose was supported by the literature. High dose differentiated from low dose iron, as discussed by Kshirsagar et al (2013) which described high dose iron as doses greater than 200mg in a one month period, and explored the safety and tolerability of ‘high dose iron sucrose’, with doses ‘7mg/kg but not exceeding 500mg’. Hence Rienso could be described as a high dose iron, as 510mg of iron was administered with each dose. Takeda denied a breach of Clause 7.2.
Takeda noted the complainant’s statement that Monofer could be given at doses of 500mg in haemodialysis patients, and 20mg/kg otherwise and that in that context Rienso appeared to be low dose. Takeda submitted that as clarified above, iron doses around 500mg were described in the literature as ‘high dose’ and it was within that context that Takeda had described Rienso as ‘high dose’ and not in the context described by the complainant whose view was inconsistent with the literature.
Takeda further submitted that, as discussed above, the term ‘high dose’ within the advertisement was intended to be read in the context of ‘Short course, High dose, Rapid bolus injection’. Rienso was only administered as a bolus injection and administration via an infusion was not described in the SPC. Conversely, Monofer offered a 20mg/kg infusion. A comparison between Rienso and Monofer’s 20mg/ kg infusion dose would not be appropriate, and this comparison was not made in the advertisement. Takeda denied a breach of Clause 7.2.
PANEL RULING
The Panel noted that the complainant was anonymous and non-contactable. As stated in the introduction to the Constitution and Procedure, anonymous complaints were accepted and like all complaints, judged on the evidence provided by the parties. Complainants had the burden of proving their complaint on the balance of probabilities.
The Panel noted the complainant’s submission that the advertisement described Rienso as ‘high dose’ but did not state what this was compared to. Takeda submitted that ‘high dose’ was used in conjunction with ‘Short course’ and ‘Rapid bolus injection’ to describe the attributes of Rienso’s administration and was not used to compare Rienso to other medicines. The Panel did not consider that the use of ‘high dose’ in this context was a hanging comparison. No breach of Clause 7.2 was ruled.
The Panel noted the complainant’s further allegation that Rienso appeared to be low dose in the context of Monofer which could be given at doses of 500mg in haemodialysis patients and 20mg/kg otherwise. The Panel noted that literature provided by Takeda described high dose iron as doses greater than 200mg in a one month period. In the Panel’s view the description of Rienso as high dose was supported by the literature. The Panel ruled no breach of Clause 7.2 in that regard.
B WEBSITE
COMPLAINT
The complainant noted that on the www.anaemiazone.co.uk website Rienso was described as a new IV iron whereas it had been available for over a year. The site stated that both the structure was designed to allow rapid administration of high doses. The complainant alleged that this was unlikely because Rienso appeared to cause more side effects than other IV irons especially immunological reactions and that the high dose was also incorrect. Section 3 stated that Monofer took five injections for 1g whereas it only took two. Section 3 further stated that all IV irons were contraindicated in hypersensitivity to Rienso or other iron preparations. The complainant alleged that this was only true for Rienso. The complainant further alleged that the cost effectiveness section only took into account the cost of the medicine and not the administration cost and was therefore misleading and unfair. The ‘Why Rienso?’ section repeated that Rienso was new which was incorrect. The complainant alleged that the claim that Rienso was convenient was very debatable in non haemodialysis patients as three other preparations only required one infusion. Finally, the cost-competitive statement was repeated although only the medicine cost was referred to. The complainant asked that the Authority look into the matter as there were several things that were of concern. The complainant submitted that he/she did not have the time to review the references in detail but considered that a more detailed review should be undertaken as there were so many issues identified upon a superficial review.
When writing to Takeda, the Authority asked it to respond in relation to Clauses 2, 7.2, 7.3, 7.4, 7.9, 7.11 and 9.1.
RESPONSE
Takeda noted that Clause 7.11 stated that new must not be used to describe any product or presentation which had been generally available, or any therapeutic indication which had been generally promoted, for more than 12 months in the UK. The Rienso SPC stated that the date of first authorization was 15 June 2012. Takeda submitted that Rienso was not generally available in the UK until the end of October 2012. In that regard Takeda provided a copy of a warehouse delivery note dated 25 October 2012 which detailed the shipment of quantities of Rienso. Takeda denied a breach of Clause 7.11.
In response to a request for further information on this point Takeda submitted that several months elapsed between the marketing authorization being granted for Rienso (15 June 2012) and Rienso being generally available following delivery to the UK wholesaler (October 2012). Takeda detailed the activities undertaken in chronological order. On 15 June 2012 Takeda gained marketing authorization for Rienso. On 23 July 2012 Takeda entered into an agreement with a named agency to manage the entry of Rienso into the UK NHS market. Relevant pages of the agreement were provided including one which detailed the scope of the agreement which was to map the relevant budget holders for budget impact modelling and to articulate Rienso’s value proposition. Takeda’s records showed that on 8 August 2012 product and therapy area training of the agency employees under the agreement of 23 July 2012 was completed. Takeda submitted that therefore the earliest that Rienso would have been promoted to any UK health professional would have been 9 August 2012. Copies of the training agenda and the corresponding certificate were provided. The full agreement with the agency was withheld. In October 2012 Rienso was delivered to the UK wholesaler and the full launch of Rienso was announced in an advertisement on 9 November 2012. The advertisement was certified on 6 November 2012 and a Rienso launch letter, certified on 13 September 2012, was distributed to health professionals on 9 November 2012.
In response to a request for further information on this point Takeda submitted that the website containing the word new had been taken down on 1 August 2013.
Takeda submitted that the construction of the particular part of the complaint wherein the complainant noted that the website stated ‘… both the structure is designed to allow rapid administration of high doses. Since Rienso appears to cause more side effects that other IV irons seems unlikely (especially immunological reactions) and the high dose is also incorrect’ was not written clearly and appeared flawed in its editing. Takeda, however, understood the complaint to challenge Rienso’s safety profile stating that there were more side effects than other IV irons. Takeda referred to its response on ‘high dose’ iron in Point A above.
Takeda was disappointed not to be able to ask the complainant to clarify what he/she meant by ‘this seems unlikely’. Takeda would have preferred to ask what this opinion was based on so that it could adequately address the specific concern.
The complainant focused on three aspects of Rienso’s safety profile: a comparison of Rienso with other IV irons; immunological reactions and concerns relating to rapid administration.
Takeda was not clear what data the complainant had used with regard to the concern about a comparison of the safety profile of Rienso ‘with other IV irons’ as this concern was not consistent with its knowledge of Rienso. Takeda stated that in its view, clear comparisons of medicines could only be made from randomised head-to-head studies.
Following three phase III studies which compared Rienso with oral iron, (Spinowitz et al 2007, Spinowitz et al 2008 and Provenzano et al 2009) and a fourth study which focussed on safety vs placebo, (Singh et al 2008), a phase II safety study was undertaken to evaluate Rienso head-to-head with IV iron sucrose (Macdougall et al 2011). Data from this head-to-head study had been presented in a poster at the American Society of Nephrology’s Kidney Week, 2011, and in a corresponding abstract. To put the results into context, one gram of IV iron was administered in the iron sucrose group (five 200mg injections if the patient was not on haemodialysis, and ten 100mg injections if the patient was on haemodialysis). In the case of Rienso, two 510mg injections were administered to all patients whether they were on haemodialysis or not. This meant, overall, patients in the iron sucrose group received five or ten exposures to iron administration, whereas in the Rienso group, patients were only exposed to two administrations of iron. The difference in the number of injections probably led to the numerical difference seen in adverse events between the two groups, as commented upon by the authors.
The iron sucrose group recorded 161 adverse events (AEs) in 53 (65%) of patients whereas 86 adverse events were experienced in 38 (48%) of patients in the Rienso group as summarised below:
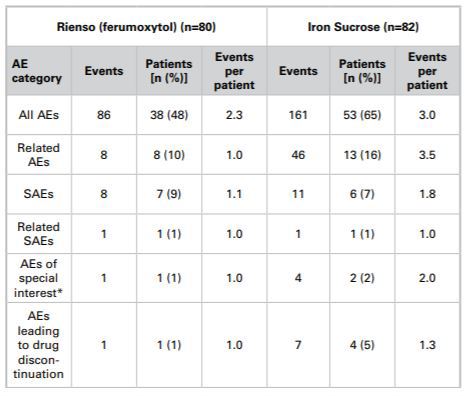
*acute moderate-to-severe acute hypotension and hypersensitivity reactions
Takeda submitted that it was clear the complainant’s statement ‘more side effects than other IV irons’ was not substantiated by this head-to-head study. With regard to the safety study, alluded to earlier (Singh et al), the authors concluded that ferumoxytol was ‘well tolerated and had a safety profile similar to placebo in anaemic patients with CKD stage 1 to 5D’, (ie patients with mild CKD through to end-stage renal disease who required treatment with haemodialysis). This was not in breach of Clause 7.9.
Takeda submitted that from the data presented, immunological reactions were captured as AEs of special interest and the results with Rienso were similar to iron sucrose.
Section 4.8 of the Rienso SPC stated that in clinical trials involving 1,562 subjects, serious hypersensitivity or hypotensive reactions were uncommon, and were reported in 3 (0.2%) of patients. One of these three cases was characterised as an anaphylactoid reaction. Also, the system organ class, immune system disorders, had hypersensitivity including anaphylaxis classified as uncommon, and life-threatening anaphylactic/ anaphylactoid reactions with a frequency that was not estimable from the available data.
The concern over the anaphylactic/anaphylactoid reactions with all IV irons had been flagged to the European Medicines Agency (EMA) by the French regulatory authority and the entire class had come under scrutiny via Article 31 of the EU Directive 2001/83/EC. This had now concluded and a report was published on 28 June 2013. In particular, for the point discussed here, all IV irons had a small risk of causing allergic reactions which could be life-threatening if not treated promptly. The recommendations also included that patients should be closely observed for signs and symptoms of hypersensitivity reactions during and for at least 30 minutes following each injection of an IV iron. This was a blanket opinion on the class of IV irons, and a distinction was not drawn between the available preparations. Takeda awaited the decision of the European Commission as to whether to make the Committee for Medicinal Products for Human Use (CHMP) recommendations legally binding across the European Union, and to learn how this might affect every respective iron SPC.
Takeda submitted that all information on the website about side effects reflected the available evidence and therefore was not in breach of Clause 7.9.
The Rienso SPC also included data from a post marketing observational study which retrospectively analysed data from over 8,600 patients who had attended three large dialysis clinics in the US. This showed that, over a 1 year period, more than 33,300 doses of Rienso were administered. Almost 50% of patients received repeat dosing with 4 or more doses. Mean haemoglobin increased by 0.5-0.9 g/ dL post-treatment and stabilised in the range of 11-11.7g/dL over the 10 month post-dose period; no new safety signals were identified with repeat dosing.
Takeda noted that Clause 7.9 stated that information and claims about side effects should be capable of substantiation by clinical experience. With the information described above from the postmarketing observational study, Takeda submitted that clinical experience substantiated the information on the website regarding side effects and therefore Takeda denied a breach of Clause 7.9.
Takeda noted the complainant’s concern regarding the safety of administering Rienso as a rapid bolus injection. Provenzano et al, in a phase III trial which compared Rienso with oral iron, explained that the body of evidence demonstrated that Rienso had an acceptable pharmacokinetic profile that allowed bolus dosing, which included lower free iron saturations than comparator irons, such as a 6-fold lower catalytically active iron concentration (bleomycin detectable free iron) than iron sucrose, Jacobs et al (2004) (abstract). Jahn et al (2011) also demonstrated low free iron concentration with Rienso.
The above information illustrated that there were no concerns about Rienso being administered as a rapid bolus injection. Also, it was true to state that within the class of IV iron administration, when comparing products’ SPCs, Rienso was indeed rapid as a 510mg dose could be administered in a minimum of 17 seconds, making it the quickest iron available to administer such a quantity in its class.
With reference to rapid administration of iron, Takeda submitted that it had not misled the reader as the information provided was accurate, balanced, fair, objective and unambiguous based upon contemporaneous data which clearly reflected all of the evidence available. Additionally the claim of rapid bolus injection was not inconsistent with the Rienso SPC.
Takeda submitted that when the website was certified, Monofer could only be administered in maximum doses of 200mg in patients on haemodialysis. The Monofer SPC had since been amended to allow a maximum of 500mg in haemodialysis patients. The updated SPC was uploaded onto the electronic Medicines Compendium (eMC) on 17 July, thirteen days before the complaint.
Takeda submitted that in addition to daily monitoring of the media and scientific journal scanning services, it adhered to a policy which required manual checking of the eMC monthly to monitor competitors’ SPCs. Takeda submitted that this was an appropriate interval. As there had been no press coverage about the change to the Monofer SPC, Takeda had not noticed the change to the competitors’ SPC in the relatively short time it took the complainant to write his/her letter as a maximum of one month had not elapsed since the SPC was updated on the eMC website.
Takeda submitted that it did not intend to mislead health professionals, whilst it acknowledged that absence of awareness was not a justification, Takeda noted that its action of immediately withdrawing the website upon hearing its competitors’ news demonstrated its commitment to the spirit of the Code. Takeda submitted that since receiving this complaint, it would check eMC twice-weekly until it received advice from the Panel on the appropriate interval for competitor surveillance. Takeda submitted that it had also withdrawn all other materials that referred to Monofer having a 200mg cap for administration in haemodialysis. Takeda considered that, despite reasonable competitor monitoring and its intention to provide factually accurate, up-to-date information without misleading the reader, the website might technically be in breach of Clause 7.2.
Takeda submitted that it had explored its competitors’ SPCs regarding warnings and precautions and contraindications when investigating the complainant’s allegation that whilst the website stated that all IV irons were contraindicated in hypersensitivity to Rienso or other iron preparations, this was only true for Rienso. Takeda noted that patient safety was of particular concern for industry and health professionals especially since every IV iron contained warnings regarding hypersensitivity or anaphylaxis/ anaphylactoid reactions. Takeda listed the contraindications relating to hypersensitivity to iron for each brand’s active substance.
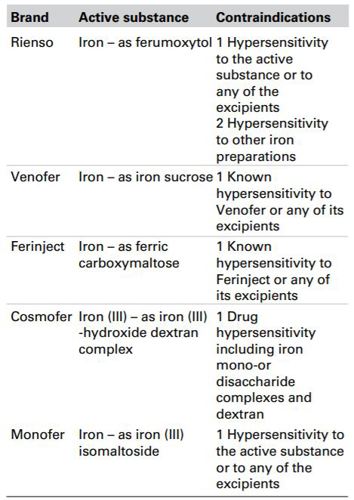
Rienso appeared to be the ‘odd one out’ where ‘hypersensitivity to other iron preparations’ was an explicit contraindication. Cosmofer included a contraindication to drug hypersensitivity including iron mono- or disaccharide complexes and dextran, some of which were in effect other iron preparations and therefore Rienso was not the only one with such a contraindication.
Takeda noted that Rienso was the only IV iron that had been granted a marketing authorization via the centralised procedure with the EMA which might indicate why it had the additional contraindication of hypersensitivity to other iron preparations.
When the website was in development, Takeda’s view was that iron was iron regardless of the brand administered. Takeda appreciated that excipients differed between brands but noted that iron was the active ingredient for each preparation and assumed that its competitors’ respective contraindications relating to hypersensitivity to iron, either described as the brand name or as an active substance meant that hypersensitivity to any iron preparation was in principle a contraindication for every brand. The recently published CHMP recommendations on how to manage the risk of allergic reactions with IV iron-containing medicines concluded that provided adequate measures to reduce the risk of allergic reactions were taken, this class of medicine had benefits that outweighed the risks. Caution was warranted with every dose of IV iron, even if previous administrations had been well tolerated. The CHMP opinion stated that ‘intravenous iron containing products were contraindicated in patients with hypersensitivity to the active substance or excipients, and intravenous iron-containing products must not be used in patients with serious hypersensitivity to other parenteral iron products’. Takeda submitted that the body of evidence appeared to point in the direction that all IV iron preparations should carry a contraindication of hypersensitivity to other IV iron preparations which was in line with its interpretation when it developed the website. The therapy area awaited the decision of the European Commission as to how the CHMP opinion should be reflected in the class’s SPCs.
Takeda submitted that the statement on its website reflected the general belief about the use of IV irons after hypersensitivity had already been experienced to one IV iron preparation as supported by the CHMP report on this matter. Takeda denied a breach of Clause 7.4.
With regard to the complainant’s allegation that the section entitled ‘cost-effectiveness’ only took into account the cost of the medicine and not the true cost to administer which was misleading and unfair, Takeda noted that it claimed that Rienso was a cost-competitive option for rapid and convenient IV iron management in CKD and substantiated it by tabulating prices. In descending order of price, compared with Ferinject and Monofer, Rienso was cheaper. Takeda noted that it could clearly not claim that Rienso was the cheapest option as Cosmofer and Venofer were cheaper and so it used ‘cost-competitive’. The voiceover did not make any additional claim. Takeda noted that the complaint was based on a discussion of the meaning of ‘cost’. The www.theefreedictionary.com defined cost as, inter alia, ‘an amount paid or required in payment for a purchase; a price’. Cost was therefore synonymous with price. The term ‘cost-effectiveness’ was used to indicate the section of the website where cost was presented. It was named in a similar fashion to Section 2, where ‘safety’ indicated where the safety data was presented without necessarily making the claim that ‘Rienso was safe’ as this was not allowed. Takeda denied that its claim of cost-competitiveness was misleading, and was not an unfair comparison as alleged.
Takeda noted that the complainant had been led to the website from a journal advertisement which included the claim ‘new SMC [Scottish Medicines Consortium] advice available’ which referred to SMC’s website where a cost minimisation analysis was discussed which led to the publication of the SMC’s advice for NHS Scotland. Takeda decided not to place the SMC advice on the website as this important information for Rienso was presented in other promotional materials that subsequently directed health professionals to the SMC website and to www.anaemiazone.co.uk. The Takeda UK website had not undergone search engine optimisation and did not appear early in the hit list when searching for Rienso. It was unlikely that the website would be read in isolation. Takeda denied breaches of Clauses 7.2, 7.3 and 7.4.
Takeda agreed that debate was needed regarding the complainant’s view that the convenience Rienso offered was ‘very debatable in non haemodialysis patients as three other preparations only require one infusion’. Takeda submitted that Rienso was convenient to administer because 1g of iron could be administered with two injections in a short course, high dose, rapid bolus injection over two to eight days. Takeda noted that the complainant appeared to consider that an infusion over a number of hours with the additional expenditure of nurse time and the use of NHS services and the ensuing observation period was more convenient than the administration of one or two rapid bolus injections which were each administered in as little as 17 seconds, with 30 minutes of post-dose observation, over two visits (if a second dose was needed) within two to eight days. Takeda disagreed with the complainant’s opinion. There were pros and cons for each side of the argument but Takeda submitted that Rienso offered a convenient option for patients and health professionals. Takeda denied a breach of the Code.
In summary, Takeda was disappointed that despite the website offering contact details and a medical information option, the complainant approached the PMCPA. Takeda submitted that despite monitoring the eMC website at monthly intervals, which demonstrated its intention to uphold the spirit of Clause 7.2, it had technically breached the Code with respect to a competitor’s SPC. Takeda noted that the complainant appeared to be very well acquainted with the competitor’s SPC and its update as the complaint was written within 13 days of the update appearing on the eMC. Takeda denied a breach of Clauses 7.3, 7.4, 7.9 and 7.11 with regards to the website.
Takeda submitted that although it did not notice an unannounced update to a competitor’s SPC despite regular surveillance, it did not consider that it had failed to maintain high standards and denied a breach of Clause 9.1. Subsequently Takeda denied a breach of Clause 2 which was reserved for circumstances where activities or materials associated with promotion had brought discredit to, and reduced of confidence in, the industry.
Takeda maintained its strong commitment to adhere to the letter and spirit of the Code and its value of the importance of the industry’s position in the wider society.
PANEL RULING
The Panel noted, with regard to the allegation that Rienso was described on the website as a new IV iron whereas it had been available for over a year, that the Rienso SPC listed 15 June 2012 as the date of first authorization. The Panel further noted Takeda’s account of its activities subsequent to that date and its submission that Rienso could not have been promoted to any UK health professional before 8 August 2012 as this was when the training of the agency’s employees was completed. The Panel noted, however, that the contract between the agency and Takeda stated that ‘[the agency] would carry out and perform the services…’ with effect from the commencement date….’ ie from 23 July. The Panel noted that the services included navigating the changing NHS in the correct timelines with the correct information (advanced product notification (APN) and budget impact model) to ensure appropriate local product update. Reference was made to engaging the right decision makers in a local health economy who planned the budget and introduction of new oncology medicines and ensure key opinion leader advocacy at launch. The advanced product notification referred to budgetary conversations that would take place with relevant NHS budget holders. The Panel noted that these activities would be carried out from 23 July ie 5-6 weeks after Rienso had received its marketing authorization. The Panel considered that such activity with Rienso was promotional. In that regard Rienso had thus been promoted since 23 July 2012 and so, to meet the requirements of the Code, could not be described as ‘new’ beyond 22 July 2013. The Panel noted Takeda’s submission, however, that the product had been described as new on the anaemiazone.co.uk website until 1 August 2013. A breach of Clause 7.11 was ruled.
The Panel noted the complainant’s view that the site stated the structure was designed to allow rapid administration of high dose but that seemed unlikely since Rienso appeared to cause more side effects than other IV irons (especially immunological reactions). The Panel understood the complainant to mean that as Rienso caused more side effects than other IV irons (especially immunological reactions) it was unlikely that the structure was designed to allow rapid administration of a high dose. The complainant did not provide any evidence to support his/her allegation. The Panel noted that the website stated that ‘The unique structure of Rienso is designed to allow rapid administration of high doses (510mg) of iron’. A bullet point below stated that the protective PSC coating shielded the bioactive iron oxide from the plasma to reduce immunological sensitivity and reduce release of free iron. The Panel noted that Section 4.8 of the Rienso SPC stated that in clinical trials involving 1,562 subjects, serious hypersensitivity or hypotensive reactions were uncommon, and were reported in 3 (0.2%) of patients with CKD who received Rienso. The Panel further noted that all of the IV iron SPCs provided by Takeda stated that parenteral administration of all iron complexes might cause immediate severe and potentially lethal hypersensitivity reactions. In the Panel’s view no evidence was provided to support the allegation that Rienso caused more side effects than other IV irons (especially immunological reactions). The Panel noted that according to the SPC, Rienso was administered as an undiluted IV injection delivered at a rate of up to 1ml/sec (30mg/sec) ie at least 17 seconds per vial. Provenzano et al stated that in vitro data suggested that ferumoxytol contained less free iron than other IV preparations and it was perhaps these physicochemical characteristics that permitted the rapid administration of larger doses of ferumoxytol compared with currently available iron preparations. The Panel considered that the statement ‘The unique structure of Rienso is designed to allow rapid administration of high doses (510mg) of iron’ was accurate, reflected the evidence and was capable of substantiation. The Panel thus ruled no breach of Clauses 7.2 and 7.4.
The Panel noted its comments at Point A above and considered that its ruling of no breach of Clause 7.2 in relation to describing Rienso as ‘high dose’ also applied to the website.
The Panel noted that Section 1 of the website (not 3 as referred to by the complainant) contained a bar chart headed ‘FEWER bolus injections to deliver 1g iron vs most other IV irons’ which showed that to deliver 1g of iron required 2 bolus injections of Rienso and 5 bolus injections of Monofer. The Panel noted Takeda’s submission that when the website was certified, Monofer injection could only be administered in maximum doses of 200mg in patients on haemodialysis but that the SPC had since been amended to allow a maximum dose of 500mg in patients on haemodialysis. The updated Monofer SPC was uploaded onto the eMC on 17 July 2013, 13 days before the complaint was submitted. Takeda had missed the update as it only monitored the eMC once a month; the company had acknowledged that the website at issue had thus included outdated information about Monofer for some days. Clause 7.2 of the Code required information and claims to be up-to-date and in that regard there was no grace period. The Panel ruled a breach of Clause 7.2. The material at issue could not be substantiated. The Panel ruled a breach of Clause 7.4.
The Panel noted the website stated that ‘As with all IV irons, the use of Rienso is contraindicated in cases of: hypersensitivity to Rienso, its excipients or other iron preparations’; the complainant alleged that this was only true for Rienso. The Panel noted Takeda’s acknowledgement that, following a comparison of its competitors’ SPCs, Rienso appeared to be the only one with hypersensitivity to other iron preparations listed as an explicit contraindication. The Panel considered that the claim thus did not reflect the available evidence and was not capable of substantiation. A breach of Clauses 7.4 and 7.9 was ruled.
The Panel noted the allegation that the cost effectiveness section of the website (Section 4) only took into account the cost of the medicine and not the true cost to administer and was therefore misleading and an unfair comparison. The Panel noted Takeda’s submission that while cost effectiveness was used to indicate the section of the website where cost was presented, Takeda had only claimed that Rienso was a cost-competitive option for rapid and convenient IV iron management. In the Panel’s view, use of the heading ‘cost effectiveness’ to describe a section of the website which only detailed acquisition cost was misleading. The table of data provided in Section 4 of the website listed the ‘Calculated NHS list price to administer 1g of IV iron’ and thus it would be clear to the reader that the costs of the five medicines cited were acquisition costs only and did not take into account any related administration costs. Nonetheless, the Panel considered that to put such data under a heading of ‘cost effectiveness’ was misleading and a breach of Clauses 7.2 and 7.3 was ruled.
With regard to the allegation that the claim that Rienso was a convenient way to deliver 1g of iron was very debatable in non haemodialysis patients, as three other preparations only required one infusion, the Panel noted Takeda’s submission that Rienso offered a convenient option to patients as well as health professionals as it allowed 1g of iron to be administered with two injections in a short course, high dose, rapid bolus injection administered in 17 seconds with 30 minutes of post-dose observation over two to eight days. On balance the Panel considered that in light of current IV iron therapy, the claim that Rienso was convenient was not misleading. In that regard, the Panel ruled no breach of Clause 7.2.
The Panel noted its rulings above and considered that high standards had not been maintained. A breach of Clause 9.1 was ruled. The Panel did not consider, however, that the material at issue was such as to bring discredit upon, or reduce confidence in, the pharmaceutical industry. No breach of Clause 2 was ruled.
Complaint received 30 July 2013
Case completed 11 October 2013


