Case Summary
A pharmacist and clinical senior lecturer, complained about a Seretide (salmeterol/fluticasone) email sent by GlaxoSmithKline via eGuidelines.co.uk. Seretide was indicated for use in patients with asthma or chronic obstructive pulmonary disease (COPD).
The heading to the email stated that Seretide now delivered even greater value to the NHS and stated that 'The price of Seretide Accuhaler 100 has been reduced by 42% to £18 and is now the same price as the Seretide Evohaler 50'. A bullet point which followed stated 'Seretide is priced competitively compared to other ICS/LABA [inhaled corticosteroids/long-acting beta-agonist] combinations at equivalent doses'. This claim was referenced to MIMS, January 2012. A second bullet point stated 'Prescribed appropriately, Seretide can help achieve NHS quality and productivity targets' and was referenced to Doull et al (2007) and Briggs et al (2010).
The complainant alleged that the claim that Seretide products were competitively priced compared with other ICS/LABA products due to a 42% decrease in price of the Seretide Accuhaler 100 was incorrect. The email did not show how the cost had been calculated other than a reference to MIMS. The complainant submitted that the cost of a Seretide inhaler was higher than all the competitor products across the whole dose range. Depending on the dose and product chosen, the variation was at least 8% and the claim was, therefore, misleading. The complainant further alleged that the claim that Seretide was priced competitively and could help the NHS quality and productivity targets could not be substantiated.
The detailed response from GlaxoSmithKline is given below.
The Panel noted that, from the information provided by GlaxoSmithKline, at low and medium doses, Seretide Accuhaler and Seretide Evohaler were the same price and neither was the most expensive nor the cheapest ICS/LABA combination available. At high dose, Seretide Accuhaler was the least expensive and Seretide Evohaler the second least expensive.
The Panel considered that the claim at issue, 'Seretide is priced competitively compared to other ICS/LABA combinations at equivalent doses', did not imply that the Seretide preparations were the least expensive combinations but rather that they were somewhere in the middle of the price range. This was the case for low and medium doses of the Seretide preparations, with the high dose preparations being the least expensive, as noted above. The Panel noted that it was clear that the comparison was with equivalent doses.
However the dose details were not given in the email. The Panel did not consider that the claim was misleading as alleged and ruled no breach of the Code. The statement was capable of substantiation and no breach of the Code was ruled.
The Panel noted that the claim 'Prescribed appropriately, Seretide can help achieve NHS quality and productivity targets', was referenced to Doull et al and Briggs et al. The Panel further noted that national guidance described the treatment of asthma as a series of steps dependent on disease severity and response to current treatment. The third step, if symptoms could not be controlled with an ICS alone was to add in a LABA. The Panel noted GlaxoSmithKline's submission that 76% of such patients were eligible for the lowest dose of Seretide (either Seretide Accuhaler 100 or Seretide Evohaler 50), yet only 20% of them received this lowest dose and subsequently a significant proportion of Seretide patients were commenced on doses that were higher than necessary. The Panel considered that it was not unreasonable to assume that reducing the cost of Seretide Accuhaler 100 could lead to cost savings.
The Panel noted that Doull et al sought to determine where in the national asthma guidance it was costeffective to use Seretide in the treatment of chronic asthma in adults and children. The authors concluded that for patients uncontrolled on beclometasone 400mcg per day or equivalent it was cost-effective to switch to Seretide compared with increasing the dose of ICS. Briggs et al reported the analysis of economic data from the Towards a Revolution in COPD (TORCH) study which aimed to inform decision makers of the potential cost-effectiveness of alternative treatments for COPD. The authors concluded that Seretide was more effective and had a lower incremental cost effectiveness ratio (compared with placebo) than either fluticasone or salmeterol alone.
The Panel noted that the cost of one presentation of Seretide had been reduced in price. Further if all presentations of Seretide were prescribed appropriately then this might help achieve NHS quality and productivity targets. The Panel did not consider that the claim 'Prescribed appropriately Seretide can help achieve NHS quality and productivity targets' was misleading as alleged and no breach of the Code was ruled. The claim was capable of substantiation and no breach of the Code was ruled.
Case AUTH/2478/2/12 PHARMACIST/CLINICAL SENIOR LECTURER v GLAXOSMITHKLINE GSK
NO BREACH OF THE CODE
Promotion of Seretide
A pharmacist and clinical senior lecturer, complained about a Seretide (salmeterol/fluticasone) email sent by GlaxoSmithKline via eGuidelines.co.uk. Seretide was indicated for use in patients with asthma or chronic obstructive pulmonary disease (COPD).
The heading to the email stated that Seretide now delivered even greater value to the NHS and stated that ‘The price of Seretide Accuhaler 100 has been reduced by 42% to £18 and is now the same price as the Seretide Evohaler 50’. A bullet point which followed stated ‘Seretide is priced competitively compared to other ICS/LABA [inhaled corticosteroids/long-acting beta-agonist] combinations at equivalent doses’. This claim was referenced to MIMS, January 2012. A second bullet point stated
‘Prescribed appropriately, Seretide can help achieve NHS quality and productivity targets’ and was referenced to Doull et al (2007) and Briggs et al (2010).
The complainant alleged that the claim that Seretide products were competitively priced compared with other ICS/LABA products due to a 42% decrease in price of the Seretide Accuhaler 100 was incorrect. The email did not show how the cost had been calculated other than a reference to MIMS. The complainant submitted that the cost of a Seretide inhaler was higher than all the competitor products across the whole dose range. Depending on the dose and product chosen, the variation was at least 8% and the claim was, therefore, misleading. The complainant further alleged that the claim that Seretide was priced competitively and could help the NHS quality and productivity targets could not be substantiated.
The detailed response from GlaxoSmithKline is given below.
The Panel noted that, from the information provided by GlaxoSmithKline, at low and medium doses, Seretide Accuhaler and Seretide Evohaler were the same price and neither was the most expensive nor the cheapest ICS/LABA combination available. At high dose, Seretide Accuhaler was the least expensive and Seretide Evohaler the second least expensive.
The Panel considered that the claim at issue, ‘Seretide is priced competitively compared to other ICS/LABA combinations at equivalent doses’, did not imply that the Seretide preparations were the least expensive combinations but rather that they were somewhere in the middle of the price range. This was the case for low and medium doses of the Seretide preparations, with the high dose preparations being the least expensive, as noted above. The Panel noted that it was clear that the comparison was with equivalent doses.
However the dose details were not given in the email. The Panel did not consider that the claim was misleading as alleged and ruled no breach of the Code. The statement was capable of substantiation and no breach of the Code was ruled.
The Panel noted that the claim ‘Prescribed appropriately, Seretide can help achieve NHS quality and productivity targets’, was referenced to Doull et al and Briggs et al. The Panel further noted that national guidance described the treatment of asthma as a series of steps dependent on disease severity and response to current treatment. The third step, if symptoms could not be controlled with an ICS alone was to add in a LABA. The Panel noted GlaxoSmithKline’s submission that 76% of such patients were eligible for the lowest dose of Seretide (either Seretide Accuhaler 100 or Seretide Evohaler 50), yet only 20% of them received this lowest dose and subsequently a significant proportion of Seretide patients were commenced on doses that were higher than necessary. The Panel considered that it was not unreasonable to assume that reducing the cost of Seretide Accuhaler 100 could lead to cost savings.
The Panel noted that Doull et al sought to determine where in the national asthma guidance it was cost effective to use Seretide in the treatment of chronic asthma in adults and children. The authors concluded that for patients uncontrolled on beclometasone 400mcg per day or equivalent it was cost-effective to switch to Seretide compared with increasing the dose of ICS. Briggs et al reported the analysis of economic data from the Towards a Revolution in COPD (TORCH) study which aimed to inform decision makers of the potential cost-effectiveness of alternative treatments for COPD. The authors concluded that Seretide was more effective and had a lower incremental cost effectiveness ratio (compared with placebo) than either fluticasone or salmeterol alone.
The Panel noted that the cost of one presentation of Seretide had been reduced in price. Further if all presentations of Seretide were prescribed appropriately then this might help achieve NHS quality and productivity targets. The Panel did not consider that the claim ‘Prescribed appropriately Seretide can help achieve NHS quality and productivity targets’ was misleading as alleged and no breach of the Code was ruled. The claim was capable of substantiation and no breach of the Code was ruled.
A pharmacist and clinical senior lecturer complained about an email sent by GlaxoSmithKline UK Limited via eGuidelines.co.uk headed ‘Seretide (salmeterol xinafoate/fluticasone propionate) now delivers even greater value to the NHS’. Seretide was indicated for use in patients with asthma or chronic obstructive pulmonary disease (COPD).
The email, which was signed by a marketing director respiratory and allergy stated that ‘The price of Seretide Accuhaler 100 has been reduced by 42% to £18 and is now the same price as the Seretide Evohaler 50’. A bullet point which followed stated ‘Seretide is priced competitively compared to other ICS/LABA [inhaled corticosteroids/long-acting beta-agonist] combinations at equivalent doses’. This claim was referenced to MIMS, January 2012. A second bullet point stated ‘Prescribed appropriately, Seretide can help achieve NHS quality and productivity targets’ and was referenced to Doull et al (2007) and Briggs et al (2010). The email had been sent to GPs, pharmacists, medicines management professionals and healthcare managers.
COMPLAINT
The complainant alleged that the claim that Seretide products were competitively priced compared with other ICS/LABA products due to a 42% decrease in price of the Seretide Accuhaler 100 was incorrect. The email did not show how the cost had been calculated other than a reference to MIMS. The complainant submitted that the cost of a Seretide inhaler was higher than all the competitor products across the whole dose range. Depending on the dose and product chosen, the variation was at least 8% and the claim was, therefore, misleading. The complainant further alleged that the claim in the circular that Seretide was priced competitively and could help the NHS quality and productivity targets could not be substantiated.
The complainant stated that his main concern was the lack of transparency in the health economic calculations and the deductions implied there from. The complainant considered that an explanation as to how the claims made could be achieved would have been helpful.
When writing to GlaxoSmithKline, the Authority asked it to respond in relation to Clauses 7.2 and 7.4 of the Code.
RESPONSE
GlaxoSmithKline submitted that the email informed recipients that the price of the Seretide Accuhaler 100 had been reduced by 42%, from £31.19 to £18, on 1 January 2012. The equivalently dosed Seretide Evohaler 50 also cost £18 and so the Accuhaler and the Evohaler in this dose category were now the same price.
This cost reduction was a simple calculation and GlaxoSmithKline considered that it was presented in a clear, fair and balanced manner and was not therefore in breach of Clauses 7.2 or 7.4 of the Code.
With regard to the claim ‘Seretide is priced competitively compared to other ICS/LABA combinations at equivalent doses’, GlaxoSmithKline submitted that not all inhaled corticosteroids were the same and had different potencies. The British Thoracic Society/Scottish Intercollegiate Guidelines Network (BTS/SIGN) Guideline on the Management of Asthma recommended equivalent doses of inhaled corticosteroids and compared them with another inhaled corticosteroid, beclometasone (BDP).
The corticosteroid in Seretide was fluticasone propionate which had a different potency from BDP or budesonide (the inhaled corticosteroid contained in AstraZeneca’s product Symbicort). The BDP in Chiesi’s product Fostair was characterised by an extra fine particle size distribution which resulted in a more potent effect than standard formulations of BDP.
The BTS/SIGN Guideline stated that ‘fluticasone provides equal clinical activity to both BDP and budesonide at half the dose’ and that 200mcg of BDP in Fostair was equivalent to 400mcg of standard BDP. Therefore, when comparing different inhaled corticosteroids, a Seretide 200mcg inhaler would be comparable to a 400mcg inhaler of either BDP or budesonide (the inhaled corticosteroid contained in Symbicort) or a 200mcg inhaler of Fostair.
GlaxoSmithKline provided a table setting out the various different ICS/LABA combination inhalers currently available, their dose-equivalence (at low, mid and high doses as defined by the Global Initiative for Asthma) and the 30 day cost.
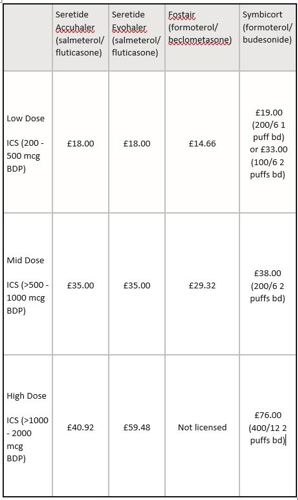
GlaxoSmithKline submitted that the table showed that, at equivalent doses, Seretide was not the most expensive combination therapy across the product range. At low and mid doses, Seretide was neither the most expensive nor the cheapest ICS/LABA combination and furthermore, for patients requiring a high-dose inhaled corticosteroid, Seretide Accuhaler 500 was the cheapest ICS/LABA combination available.
GlaxoSmithKline submitted that MIMS was recognised as an accepted source for providing the most up-to-date and accurate prices of medicines available through the NHS. In addition to specifying the prices of the individual inhalers, MIMS also provided clear and accurate information on the doses per unit and in the guidelines section the BDP dose equivalence of the different ICS/LABA combinations currently available. GlaxoSmithKline believed, therefore, that MIMS was an appropriate reference to support the claim that ‘Seretide is competitively priced compared to other ICS/LABA combinations at equivalent doses’, which was fair, balanced and capable of substantiation. GlaxoSmithKline denied breaches of Clauses 7.2 and 7.4.
GlaxoSmithKline noted that the Quality, Innovation, Productivity and Prevention (QIPP) agenda, a strategy introduced in 2009 by the Department of Health, aimed to improve the quality and delivery of NHS care and reduce costs to make £20 billion efficiency savings by 2014/15. The actual strategies adopted by local health providers depended on individual strategic health authorities and primary care trusts. The work-streams aligned to quality aimed to provide high quality care and those aligned to productivity aimed to drive efficiency savings.
GlaxoSmithKline stated that asthma treatment should be titrated to the severity of disease. The BTS/SIGN Guideline recommended a step-wise approach to management and stated that in adults who required regular preventer therapy (step 2) 400mcgs of BDP was an appropriate starting dose. If a patient was inadequately treated on an inhaled corticosteroid alone, then the guidelines recommended adding in a LABA (step 3) before increasing the dose of the steroid (ie move from a low dose steroid 400mcg to a mid-dose steroid 800mcg). The BTS/SIGN Guideline stated that:
‘A stepwise approach aims to abolish symptoms as soon as possible and to optimise peak flow by starting treatment at the level most likely to achieve this. Patients should start treatment at the step most appropriate to the initial severity of their asthma’.
GlaxoSmithKline submitted that of patients currently taking an inhaled corticosteroid alone (step 2), in whom the next recommended step would be Seretide or an equivalent ICS/LABA (step 3), 76% were eligible for the lowest dose Seretide preparations according to their current dose of steroid. However, at present, only 20% of adults with asthma moving from inhaled corticosteroid alone to Seretide were appropriately prescribed Seretide at the lowest dose (ie the Seretide Accuhaler 100 or the Seretide Evohaler 50).
GlaxoSmithKline stated that therefore, a significant proportion of new Seretide patients were commenced on doses of inhaled steroid that were higher than necessary. This was clearly not consistent with the BTS/SIGN Guideline and meant that some patients might be over-treated or at higher risk of side-effects. In addition, as the cost of Seretide increased with the dose of steroid, the prescription of doses of Seretide that were higher than necessary had cost implications.
Before January 2012, the Seretide Accuhaler 100 was £31.19 which was more than the equivalently dosed Seretide Evohaler 50 which cost £18 and was also similar in price to the mid-dose Seretide options which both cost £35. By reducing the price of the Seretide Accuhaler 100 to £18, GlaxoSmithKline considered that health professionals might be encouraged, where appropriate, to initiate asthma patients on the lowest dose of Seretide.
The appropriate prescribing of Seretide in asthma might therefore allow health providers to increase adherence with the BTS/SIGN Guideline by starting appropriate patients on the low dose (improving the quality of treatment). This would also reduce prescribing costs (thereby increasing productivity).
GlaxoSmithKline submitted that in the peer-reviewed studies referenced in the email, Seretide was shown to be a cost-effective treatment vs increasing the dose of fluticasone propionate (inhaled steroid alone) in asthma and vs the long-acting beta-agonist salmeterol in COPD.
An asthma example of a QIPP case study on the QIPP website was ‘Primary care asthma management to reduce costs and improve outcomes’ which focused on improving care for respiratory patients, implementing effective guideline driven prescribing and increased use of combination inhalers where appropriate whilst reducing admissions, referrals and respiratory prescribing costs.
The cost effectiveness study by Doull et al in asthma was driven by a systematic review and meta-analysis of 14 large randomised, control trials. It modelled resource utilisation by extrapolating the degree of symptoms to the likelihood of the utilisation of healthcare resource (such as GP visits or hospital admissions). Doull et al demonstrated that Seretide could reduce healthcare resource in a cost-effective manner compared with increasing the dose of inhaled steroid alone, which might help the NHS to achieve QIPP quality and productivity targets.
In addition, the National Institute for Health and Clinical Excellence (NICE) performed a technology assessment in 2008 for inhaled corticosteroids in the treatment of chronic asthma in adults and children aged 12 years and over. In Section 4.3.10, NICE stated that ‘The Committee considered that treatment with an inhaled corticosteroid plus a long-acting beta-agonist in a single combination device was at least as effective as using the same ingredients in separate devices’. In Section 4.3.11, NICE stated further that ‘The Committee also considered that, in people for whom inhaled corticosteroid plus long-acting beta-agonist treatment is appropriate, the least costly delivery method should be used, which is currently a combination device’.
In summary, GlaxoSmithKline believed that the appropriate prescription of Seretide, such as initiating appropriate patients with asthma on the Seretide Accuhaler 100, and the evidence that Seretide was cost-effective when appropriately prescribed could substantiate the claim, ‘Prescribed appropriately, Seretide can help achieve NHS quality and productivity targets’.
GlaxoSmithKline considered that the claim was fair, balanced and could be substantiated; it denied breaches of Clauses 7.2 and 7.4.
PANEL RULING
The Panel noted that a table in the BTS/SIGN Guideline indicated that 400mcg of BDP was equivalent to 200mcg of Fostair, 200mcg of Seretide and 400mcg of Symbicort. These dose equivalencies were also summarized in a table in MIMS. Both publications also stated that ‘These dosage equivalents are approximate and will depend on other factors such as inhaler technique’. The Panel further noted that the Global Initiative for Asthma publication ‘Global Strategy for Asthma Management and Prevention’ defined a low daily dose of BDP as 200-500mcg, medium daily dose as >500-1000mcg and a high daily dose as >10002000mcg.
The Panel noted that the Seretide Accuhaler was available in three strengths; 100mcg, 250mcg and 500mcg, all of which were to be administered as one inhalation twice a day. Fostair was available as a 100mcg preparation (to be administered as one or two inhalations twice daily) and Symbicort as 100mcg and 200mcg preparations (to be administered as one or two inhalations twice daily) and a 400mcg preparation (to be administered as one inhalation twice daily).
Given the dose definitions above and the information submitted by GlaxoSmithKline, the Panel noted that the 30 day cost of treatment at equivalent doses with low dose ICS/LABA combination was £18 for Seretide Accuhaler, £18 for Seretide Evohaler, £14.66 for Fostair and either £19 or £33 for Symbicort (depending on whether the 100mcg or 200mcg preparation was used). The 30 day treatment cost at equivalent doses for medium dose ICS/LABA was £35 for Seretide Accuhaler, £35 for Seretide Evohaler, £29.32 for Fostair and £38 for Symbicort. The 30 day treatment cost at equivalent doses for high doses of these medicines was £40.92 for Seretide Accuhaler, £59.48 for Seretide Evohaler and £76 for Symbicort. Fostair was not licensed above 400mcg daily (the dose equivalent of 800mcg BDP).
The Panel noted that at low and medium doses, both Seretide preparations were the same price and neither was the most expensive nor the cheapest ICS/LABA combination available. At high dose, Seretide Accuhaler was the least expensive and Seretide Evohaler the second least expensive.
The Panel considered that the claim at issue, ‘Seretide is priced competitively compared to other ICS/LABA combinations at equivalent doses’, did not imply that the Seretide preparations were the least expensive combinations but rather that they were somewhere in the middle of the price range. This was the case for low and medium doses of the Seretide preparations, with the high dose preparations being the least expensive, as noted above. The Panel noted that it was clear that the comparison was with equivalent doses. However the dose details were not given in the email. The Panel did not consider that the claim was misleading as alleged and ruled no breach of Clause 7.2. The statement was capable of substantiation and no breach of Clause 7.4 was ruled.
In relation to the claim ‘Prescribed appropriately, Seretide can help achieve NHS quality and productivity targets’, the Panel noted that the aim of the Department of Health’s QIPP agenda was to improve the quality of care delivered by the NHS whilst making up to £20 billion of efficiency savings by 2014-15. The Panel noted that the references given for the claim at issue were Doull et al and Briggs et al. The Panel further noted that the BTS/SIGN Guideline described the treatment of asthma as a series of steps dependent on disease severity and response to current treatment. The third step, if symptoms could not be controlled with an ICS alone was to add in a LABA. The Panel noted GlaxoSmithKline’s submission that 76% of such patients were eligible for the lowest dose of Seretide (either Seretide Accuhaler 100 or Seretide Evohaler 50), yet only 20% of them received this lowest dose and subsequently a significant proportion of Seretide patients were commenced on doses that were higher than necessary. The Panel considered that it was not unreasonable to assume that reducing the cost of Seretide Accuhaler 100 could lead to cost savings.
It was difficult to see that lowering the cost of Seretide Accuhaler 100 would necessarily encourage health professionals to use lower doses as submitted by GlaxoSmithKline. There was nothing in the email to encourage health professionals to consider this point.
The Panel noted that Doull et al sought to determine where in the BTS/SIGN asthma guidance it was cost effective to use Seretide in the treatment of chronic asthma in adults and children. The authors concluded that for patients uncontrolled on BDP 400mcg per day or equivalent it was cost-effective to switch to Seretide compared with increasing the dose of ICS. Briggs et al reported the analysis of economic data from the Towards a Revolution in COPD (TORCH) study which aimed to inform decision makers of the potential cost effectiveness of alternative treatments for COPD. The authors concluded that Seretide was more effective and had a lower incremental cost-effectiveness ratio (compared with placebo) than either fluticasone or salmeterol alone.
The Panel noted that the cost of one presentation of Seretide had been reduced in price. Further if all presentations of Seretide were prescribed appropriately then this might help achieve NHS quality and productivity targets. The Panel did not consider that the claim ‘Prescribed appropriately Seretide can help achieve NHS quality and productivity targets’ was misleading as alleged and no breach of Clause 7.2 was ruled. The claim was capable of substantiation and no breach of Clause 7.4 was ruled.
Complaint received 7 February 2012
Case completed 18 April 2012

