Case Summary
Novartis complained that an Arimidex (anastrozole) mailing issued by AstraZeneca presented an oversimplified and misleading cost comparison which failed to compare like with like in terms of the indications. The mailing featured a table comparing the 28 day cost of three aromatase inhibitors in the treatment of breast cancer: Arimidex 1mg (£65.56); letrozole 2.5mg (Novartis’ product Femara) (£83.16) and exemestane 25mg (Pharmacia’s product Aromasin) (£82.88).
The indications for Arimidex were:
‘Treatment of advanced breast cancer in postmenopausal women. Efficacy has not been demonstrated in oestrogen receptor negative patients unless they had a previous positive clinical response to tamoxifen.
Adjuvant treatment of postmenopausal women with hormone receptor positive early invasive breast cancer.
Adjuvant treatment of early breast cancer in hormone receptor positive postmenopausal women who have received 2 to 3 years of adjuvant tamoxifen.’
The indications for Femara were:
‘Adjuvant treatment of postmenopausal women with hormone receptor positive invasive early breast cancer.
Treatment of early invasive breast cancer in postmenopausal women who have received prior standard adjuvant tamoxifen therapy.
First-line treatment in postmenopausal women with advanced breast cancer.
Advanced breast cancer in postmenopausal women in whom tamoxifen or other anti-oestrogen therapy has failed.
Pre-operative therapy in postmenopausal women with localised hormone receptor positive breast cancer, to allow subsequent breast-conserving surgery in women not originally considered candidates for breast-conserving surgery. Subsequent treatment after surgery should be in accordance with standard of care.’
The indications for Aromasin were:
‘In patients with early breast cancer, treatment with Aromasin should continue until completion of five years of combined sequential adjuvant hormonal therapy (tamoxifen followed by Aromasin), or earlier if tumour relapse occurs.
In patients with advanced breast cancer, treatment with Aromasin should continue until tumour progression is evident.’
Given these differences Novartis alleged that it was misleading to make a cost comparison without specifying what indications were being referred to.
The Panel noted that the indications for the products differed. When Arimidex was used in accordance with its licence it would be less expensive than the other products listed when they were also so licensed. However the cost comparison appeared beneath a general heading relating to the treatment of breast cancer. Letrozole was licensed for two indications (pre-surgery treatment and following five years of tamoxifen therapy post-surgery) for which Arimidex was not. There was no information stating that the indications differed. The Panel considered that the item was a misleading
CASE AUTH/1894/10/06 NOVARTIS v ASTRAZENECA
Arimidex mailing
Novartis complained that an Arimidex (anastrozole) mailing issued by AstraZeneca presented an oversimplified and misleading cost comparison which failed to compare like with like in terms of the indications. The mailing featured a table comparing the 28 day cost of three aromatase inhibitors in the treatment of breast cancer: Arimidex 1mg (£65.56); letrozole 2.5mg (Novartis’ product Femara) (£83.16) and exemestane 25mg (Pharmacia’s product Aromasin) (£82.88).
The indications for Arimidex were:
‘Treatment of advanced breast cancer in postmenopausal women. Efficacy has not been demonstrated in oestrogen receptor negative patients unless they had a previous positive clinical response to tamoxifen.
Adjuvant treatment of postmenopausal women with hormone receptor positive early invasive breast cancer.
Adjuvant treatment of early breast cancer in hormone receptor positive postmenopausal women who have received 2 to 3 years of adjuvant tamoxifen.’ The indications for Femara were:
‘Adjuvant treatment of postmenopausal women with hormone receptor positive invasive early breast cancer.
Treatment of early invasive breast cancer in postmenopausal women who have received prior standard adjuvant tamoxifen therapy.
First-line treatment in postmenopausal women with advanced breast cancer.
Advanced breast cancer in postmenopausal women in whom tamoxifen or other anti-oestrogen therapy has failed.
Pre-operative therapy in postmenopausal women with localised hormone receptor positive breast cancer, to allow subsequent breast-conserving surgery in women not originally considered candidates for breast-conserving surgery. Subsequent treatment after surgery should be in accordance with standard of care.’
The indications for Aromasin were:
‘In patients with early breast cancer, treatment with Aromasin should continue until completion of five years of combined sequential adjuvant hormonal therapy (tamoxifen followed by Aromasin), or earlier if tumour relapse occurs.
In patients with advanced breast cancer, treatment with Aromasin should continue until tumour progression is evident.’
Given these differences Novartis alleged that it was misleading to make a cost comparison without specifying what indications were being referred to.
The Panel noted that the indications for the products differed. When Arimidex was used in accordance with its licence it would be less expensive than the other products listed when they were also so licensed. However the cost comparison appeared beneath a general heading relating to the treatment of breast cancer. Letrozole was licensed for two indications (pre-surgery treatment and following five years of tamoxifen therapy post-surgery) for which Arimidex was not. There was no information stating that the indications differed. The Panel considered that the item was a misleading comparison and a breach of the Code was ruled.
Novartis Pharmaceuticals UK Ltd complained about a cost comparison mailing (ref ARIM 06 18944) for Arimidex (anastrozole) issued by AstraZeneca UK Limited. The mailing featured a table comparing the 28 day cost of three aromatase inhibitors in the treatment of breast cancer: Arimidex 1mg (£65.56); letrozole 2.5mg (Novartis’ product Femara) (£83.16) and exemestane 25mg (Pharmacia’s product Aromasin) (£82.88).
COMPLAINT
Novartis alleged that the cost comparison was oversimplified and presented a misleading impression of the relative costs of the products and failed to compare like with like in terms of the indications as required by the Code. A breach of Clause 7.2 was alleged.
The licensed indications for the three products included in the cost comparison were not the same. The indications for Arimidex were:
‘Treatment of advanced breast cancer in postmenopausal women. Efficacy has not been demonstrated in oestrogen receptor negative patients unless they had a previous positive clinical response to tamoxifen.
Adjuvant treatment of postmenopausal women with hormone receptor positive early invasive breast cancer.
Adjuvant treatment of early breast cancer in hormone receptor positive postmenopausal women who have received 2 to 3 years of adjuvant tamoxifen.’ (Arimidex summary of product characteristics (SPC)). The indications for Femara were:
‘Adjuvant treatment of postmenopausal women with hormone receptor positive invasive early breast cancer.
Treatment of early invasive breast cancer in postmenopausal women who have received prior standard adjuvant tamoxifen therapy.
First-line treatment in postmenopausal women with advanced breast cancer.
Advanced breast cancer in postmenopausal women in whom tamoxifen or other anti-oestrogen therapy has failed.
Pre-operative therapy in postmenopausal women with localised hormone receptor positive breast cancer, to allow subsequent breast-conserving surgery in women not originally considered candidates for breast-conserving surgery. Subsequent treatment after surgery should be in accordance with standard of care.’ (Femara SPC).
The indications for Aromasin were:
‘In patients with early breast cancer, treatment with Aromasin should continue until completion of five years of combined sequential adjuvant hormonal therapy (tamoxifen followed by Aromasin), or earlier if tumour relapse occurs.
In patients with advanced breast cancer, treatment with Aromasin should continue until tumour progression is evident.’ (Aromasin SPC).
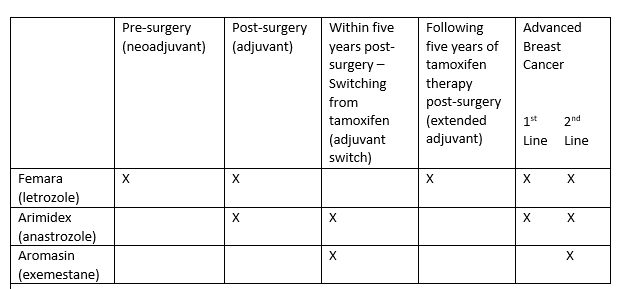
The different indications were summarised in the table below.
|
|
Pre-surgery (neoadjuvant)
|
Post-surgery (adjuvant)
|
Within five years post-surgery –Switching from tamoxifen (adjuvant switch)
|
Following five years of tamoxifen therapy post-surgery (extended adjuvant)
|
Advanced Breast Cancer
1st 2nd
Line Line
|
|
Femara (letrozole)
|
X
|
X
|
|
X
|
X X
|
|
Arimidex (anastrozole)
|
|
X
|
X
|
|
X X
|
|
Aromasin (exemestane)
|
|
|
X
|
|
X
|
Given the differences between the products, Novartis alleged that it was misleading to make a cost comparison without specifying what indications were being referred to. This was a misleading comparison in breach of Clause 7.2.
RESPONSE
AstraZeneca stated that the mailer in question was prepared in June 2006 and its primary purpose was to compare the acquisition costs per 28 days’ treatment with anastrozole (Arimidex), letrozole or exemestane. The cost comparison was based on the June 2006 issue of MIMS. These three aromatase inhibitors were normally prescribed to prevent breast cancer recurrence.
The item was sent to hospital pharmacists and network pharmacists (the latter had responsibilities in the delivery of agreed cancer action plans for the local cancer network). Cancer action plans were based on evidence based treatment strategies and evaluation of costs. As such, cancer network pharmacists were a small, specialized group responsible for clinical and budgetary planning across a larger geographical region; currently there were 34 networks covering England; hospital trusts often looked to cancer networks to advise them on such matters.
The mailer contained two standalone items: one about a recently acquired licensed indication for Arimidex and one about the acquisition costs of anastrozole, letrozole and exemastane. Acquisition costs were important because they allowed pharmacists to make informed decisions that impacted on drug-purchasing budgets thus optimising limited healthcare resources.
The item at issue clearly stated in the prescribing information the licensed indications for Arimidex. Both exemestane and letrozole shared common indications with Arimidex. As such the item at issue aimed to compare the cost of Arimidex with letrozole for the ‘Adjuvant treatment of postmenopausal women with hormone receptor positive early invasive breast cancer’ and with exemestane for the ‘Adjuvant treatment of early breast cancer in hormone receptor positive postmenopausal women who have received 2 to 3 years of adjuvant tamoxifen’. Finally all three products shared common licence indications in the advanced breast cancer setting.
AstraZeneca knew that under the Code price comparisons could only be made where like was compared with like. This requirement had been met because the dosage and dosage frequency (one tablet daily) of each product, as shown in the mailer, did not change across indications. This meant that the cost per 28 days’ treatment for each product was as shown in the mailer. Furthermore, the acquisition costs for each of the three products compared over 28 days was appropriate because treatment typically lasted months to years rather than days or weeks, eg treatment of advanced breast cancer with aromatase inhibitors typically lasted for months, whereas treatment of early breast cancer would typically be for up to five years.
AstraZeneca also recognised that price comparisons should be made on the basis of the equivalent dosage requirement for the same indication. This requirement was not relevant in this case because regardless of the specific indications for each of the three products, usage rates were identical for 28 days’ treatment: patients who were treated with any of the three aromatase inhibitors would have to take one tablet daily. Moreover, as no aromatase inhibitor had been shown to be superior over another in terms of efficacy, reducing treatment duration, improving patient compliance or improving adverse event profiles, the only valid price comparison was the one shown in the mailer, ie direct acquisition costs.
Therefore, AstraZeneca denied a breach of Clause 7.2 given the reasons outlined above.
PANEL RULING
The Panel noted that the material at issue sent to hospital and network pharmacists included the costs of 28 days’ treatment with Arimidex (£68.56), letrozole (£83.16) and exemestane (£82.88) beneath the heading ‘Comparing the cost of Aromatase Inhibitors in the treatment of breast cancer’.
The Panel noted that the indications for the products differed. When Arimidex was used in accordance with its licence it would be less expensive than the other products listed when they were also so licensed. However the cost comparison appeared beneath a general heading relating to the treatment of breast cancer and Arimidex was not the least expensive medicine for the treatment of all types of breast cancer. Letrozole was licensed for two indications (pre-surgery treatment and following five years of tamoxifen therapy post-surgery) for which Arimidex was not. There was no information stating that the indications differed. The Panel considered that the item was a misleading comparison and a breach of Clause 7.2 of the Code was ruled.
Complaint received 6 October 2006
Case completed 29 November 2006

